2 Ventricles and Cisterns
The embryologic development of the ventricles begins with three expansions (primary vesicles) of the rostral neural tube (weeks 4–5 of gestation), which are referred to as the forebrain (prosencephalon), midbrain (mesencephalon), and hindbrain (rhombencephalon). The primary vesicles subsequently expand and bend with localized constrictions to form the five secondary vesicles (approximately week 7 of gestation). The forebrain gives rise to the telencephalon (eventual cerebral hemispheres and lateral ventricles) and diencephalon (thalamus, hypothalamus, and third ventricle). The midbrain eventually forms the secondary vesicle, also referred to as the mesencephalon, which eventually forms the tectum, midbrain portion of the brainstem, and cerebral aqueduct. The hindbrain gives rise to the metencephalon (eventual pons, cerebellum, and upper portion of the fourth ventricle) and myelencephalon (eventual medulla and lower portion of the fourth ventricle).
Abnormalities in development of the cerebral vesicles result in congenital anomalies, such as the holoprosencephalies, lissencephaly/pachygyria, and Dandy-Walker malformations. Abnormalities in the closure of the caudal neural tube with altered internal pressure dynamics have been proposed as a mechanism in the malformation of the ventricles and other anomalies associated with Chiari II malformations.
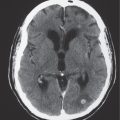
The normal lateral ventricles are bilateral elongated C-shaped structures, each containing a contiguous frontal horn, body, atrium (trigone), occipital horn, and temporal horn. The lateral ventricles are often symmetric, but varying degrees of asymmetry are not uncommon. The anterior portions of the lateral ventricles are normally separated by the septum pellucidum.
The third ventricle appears as a slitlike compartment filled with cerebrospinal fluid (CSF) between the thalami. The inferior border of the third ventricle is the hypothalamus, and the upper border is the choroid tela (fusion of the pia and ependymal lining of ventricle) and choroid plexus. The anterior border is the lamina terminalis and anterior commissure. The posterior border includes the pineal gland and recess, as well as the posterior commissure. The third ventricle communicates with the lateral ventricles via the foramina of Monro located anterolater-ally. The third ventricle communicates with the fourth via the cerebral aqueduct posteroinferiorly.
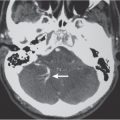
The fourth ventricle has a pyramidal shape in the sagittal plane and an inverted C shape/inverted kidney bean shape in the axial plane. The fourth ventricle is located dorsal to the pons with its roof comprised of the cerebellar vermis. It communicates with the cerebral aqueduct at its upper margin, the cisterna magna of the subarachnoid space, via the foramen of Magendie and the paired foramina of Luschka.
CSF fills the ventricles and is produced by the choroid plexus located within the lateral, third, and fourth ventricles, as well as the foramina of Luschka and Magendie. The choroid plexus typically enhances after intravenous contrast administration because of its lack of a blood–brain barrier. CSF from the ventricles communicates with the subarachnoid space adjacent to the brain and spinal cord through the foramina of Luschka and Magendie. The primary function of CSF is to protect the brain and spinal cord from trauma and rapid changes in venous pressure. It represents ~10% of the intracranial and intraspinal spaces. A total of ~150 mL of CSF is present within the ventricles and intracranial and spinal subarachnoid spaces. The choroid plexus forms 500 mL of CSF daily, allowing turnover four or five times daily. More than 90% of the CSF is normally resorbed by arachnoid villi or granulations (grouping of villi) that penetrate the dura, with resultant emptying of fluid into the intracranial venous sinuses. The remaining small amount of fluid is resorbed through the ependymal linings of the ventricles.
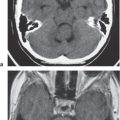
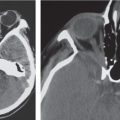
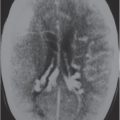
Obstruction of outflow of CSF from the ventricles results in dilation of the ventricles proximal to the site of blockage. The obstruction can result from congenital malformations (e.g., Chiari II), neoplasms/other intracranial mass lesions (e.g., colloid cyst), inflammatory lesions, hemorrhage, and brain edema/swelling (ischemia and trauma). In addition to ventricular dilation, transependymal leakage of fluid can be seen with computed tomography (CT). Obstructive or noncommunicating hydrocephalus can result, if untreated, in abnormal increased intracranial pressure, intracranial herniation, and death.
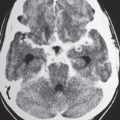
Communicating hydrocephalus occurs when there is overproduction of CSF (choroid plexus papilloma/carcinoma), impaired resorption of CSF through the arachnoid villi, and/or obstruction of CSF flow through the cisterns and sulci. With communicating hydrocephalus, the ventricles are disproportionately more prominent than the sulci. Subependymal edema may be seen with CT due to the impaired resorption of CSF. Patients with communicating hydrocephalus (normal pressure hydrocephalus) may also have clinical features of gait disturbance, incontinence, and/or progressive impairment of mental function.
Ventricular enlargement can also result from cerebral infarction, cerebral atrophy, or various neurodegenerative diseases. With these disorders, sulcal prominence is usually evident with CT.
Sulci normally vary in size, although typically they increase in size with aging. Sulcal enlargement can also be seen in a child with dehydration. Congenital malformations such as lissencephaly and pachygyria result in the absence of sulci or few shallow sulci, respectively. Sulci may be asymmetrically prominent at sites of prior cerebral or cerebellar infarction, prior intra-axial hemorrhage, contusion, inflammation, and radiation injury.
The basal cisterns represent the subarachnoid compartment adjacent to the pial margins of the inferior portions of the brain and brainstem. The cisterns are named according to the adjacent neural structures. The larger of the cisterns include the cisterna magna (dorsal and inferior to the cerebellar vermis) and superior cerebellar cistern.
Approximately 10% of neoplasms in the central nervous system extend into or are completely within the ventricles. The age of the patient and the location of the tumor influence the differential diagnosis of lesions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree