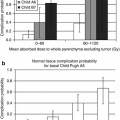
Fig. 6.1
A 2-year-old patient with retrovesical neuroblastoma (e: MRI, yellow arrow) underwent 123I-mIBG scan, for evaluation of treatment possibility with 131I-mIBG. (a) Planar scan was inconclusive because of bladder activity. (b–d) Exact localization, differentiation of the bladder and evaluation of mIBG uptake were made possible only by SPECT/CT (yellow arrows). A Foley catheter was used for emptying the bladder
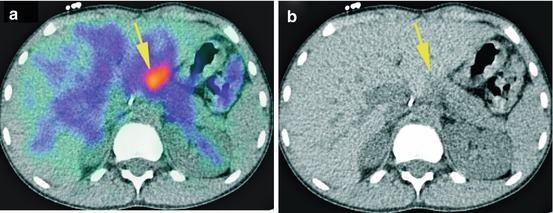
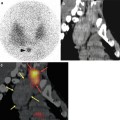
Fig. 6.2
A 9-year-old patient with stage IV neuroblastoma received 123I-mIBG scan for restaging after chemotherapy. SPECT/CT revealed a suspected uptake beside the liver (yellow arrow), which had been overlooked by MRI. The patient underwent an operation. Histopathology showed a lymph node metastasis. (a) SPECT/CT transversal view, (b) CT transversal view
6.16.2 False-Positive Findings
False-positive findings are because of contamination, most often urine contamination or any other contamination (salivary secretion). Using SPECT/CT can avoid misinterpretation of these findings.
6.17 SPECT/CT: Reducing False-Negative and False-Positive Results
As mentioned, SPECT/CT allows better anatomical localization of mIBG-avid findings, which is essential in most cases. In particular, fusion of SPECT with other imaging modalities (MRI/diagnostic CT) improves diagnostic accuracy, while low-dose CT of mIBG SPECT/CT serves as anatomical orientation [28] (Fig. 6.3).
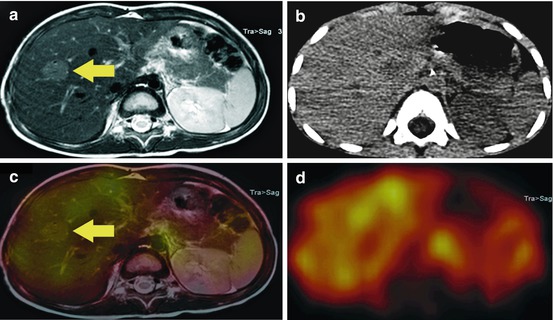

Fig. 6.3
A 3-year-old patient with suspected, new occurred liver lesion in MRI (a, yellow arrow), which was detected in his restaging examination after chemotherapy. The lesion showed no pathological 123I-mIBG uptake (c, yellow arrow and d), reported as benign, which was confirmed by biopsy as FNH. A fusion with MRI (c) was possible with the help of the low-dose CT (b) of the SPECT/CT
Detection of small foci, which would have been mostly overlooked with planar imaging alone, is possible by using SPECT/CT (Fig. 6.4).
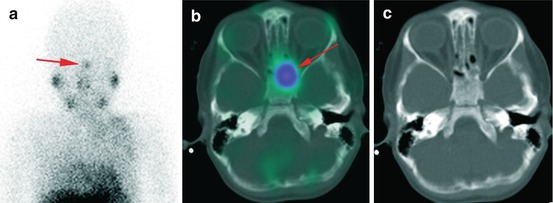

Fig. 6.4
A 4-year-old patient with tracer accumulation in the region of nasal mucosa (a, red arrow). SPECT/CT (b, c) revealed sphenoid bone involvement confirmed with MRI. This result resulted in further chemotherapy
6.18 MIBG SPECT/CT in Phaeochromocytoma and Paraganglioma
The phaeochromocytoma is a catecholamine-secreting tumour of the chromaffin cells, and it is localized in the adrenal medulla to 85 % and occurs with an incidence of 1/100,000 people p.a.
Its characteristic symptoms are episodic headaches, palpitations, diaphoresis and paroxysmal or sustained hypertension [7]. The phaeochromocytoma occurs in isolation or as part of a syndrome, e.g. MEN2 syndrome, von Hippel-Lindau syndrome or neurofibromatosis type 1 (M. Recklinghausen). With widespread use of cross-sectional imaging, an increasing number of phaeochromocytomas are diagnosed incidentally, without the presence of symptoms or complaints by the patient. Incidental tumours, as well as tumours detected while screening patients with hereditary syndromes, tend to be smaller than symptomatic ones.
The first diagnostic method is plasma or urine measurements of catecholamines and their metabolites [7, 47]. If there is positive biochemical testing, further imaging is required, such as ultrasound, CT of the abdomen or MRI.
mIBG scintigraphy for detecting phaeochromocytomas or paragangliomas has been widely used for more than 25 years and has a reported high sensitivity and specificity of about 83–100 % and 85–100 %, respectively [48]. In the last 5 years, however, image quality and spatial resolution of other imaging methods, such as MRI with a high sensitivity for detection of tumours in the adrenal gland (which are usually hyperintense on T2-weighted images and hypointense on T1-weighted images), increased significantly [7, 49]; in addition, other functional imaging modalities such as 18F-DOPA PET/CT have been increasingly applied. Regarding this, some studies have been published that compared sensitivity and specificity of those other imaging methods to mIBG SPECT. They all share a significantly lower sensitivity for mIBG scintigraphy, from which it might be concluded that the mIBG scan has lost its monopoly position as the gold standard functional imaging method for phaeochromocytoma/paraganglioma. Critics find fault in its low spatial resolution, the long examination time of 24 h, the necessity of thyroid blockade, the interference with several medications and the significant tracer uptake in the normal adrenal medulla [50].
The disadvantage of the low spatial resolution may be largely compensated by the use of mIBG SPECT/CT; however, the other “disadvantages” remain. Additionally, limitations in terms of decreased sensitivity and specificity exist in several diseases, such as MEN2-related phaeochromocytoma, extra-adrenal, multiple or hereditary paragangliomas or metastatic disease, which may lead to a significant underestimation of the extent of disease in the mIBG scan with potentially inappropriate management. The particular strengths of mIBG SPECT/CT are detection of local recurrence, small extra-adrenal phaeochromocytomas, multifocal tumours or the presence of metastatic disease [27]. In patients with clinical or biochemical suspicion of phaeochromocytoma, SPECT/CT, compared to SPECT and planar imaging, has a significantly higher accuracy (Fig. 6.5) [51]. 123I-mIBG scintigraphy, precisely with assistance of SPECT/CT, can serve for evaluation and dose calculation for 131I-mIBG therapy, from which patients may benefit [7, 52, 53].
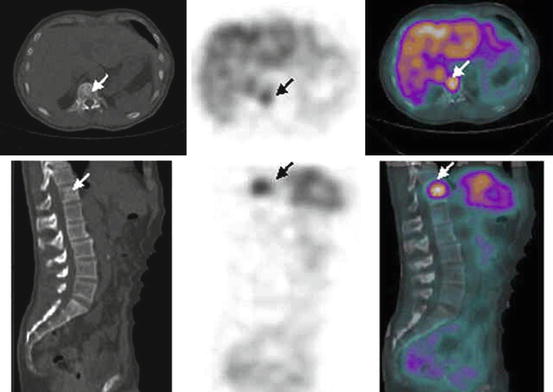

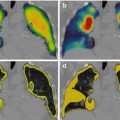
Fig. 6.5
CT, mIBG SPECT and fused mIBG SPECT/CT images of phaeochromocytoma metastasis to the body of T12 vertebra (white arrow) (With kind permission from Springer Science + Business Media: Meyer-Rochow et al. [27]. Fig. 1B)
6.18.1 Interpretation of the Findings
In some cases, there are quite unequivocal true-positive findings with a convincing focal mIBG uptake, matched to an obvious size of adrenal masses in additional imaging such as CT. These unequivocal findings can even be detected only by using whole body and planar imaging. In such cases, SPECT/CT may be useful to rule out any extra-adrenal manifestations and, otherwise, does not have any additional benefit.
Concerning less conclusive accumulations or small masses in the adrenal, low-dose CT of SPECT/CT serves as an anatomical landmark. In SPECT/CT, the left adrenal is often detected much more easily than the right adrenal due to the anatomical location of the liver and large vessels, such as vena cava, with their physiological uptake. Especially for the detection of the right adrenal, the use of SPECT/CT is very valuable; even when it only provides an inconspicuous finding, this can thus be diagnosed.
Additionally, there are several other difficulties and challenges besides localization, namely, the distinction between a significantly increased uptake, therefore interpreted as pathological, and a mildly enhanced uptake, slightly above or similar to that of the liver. This is because there are no cut-off values from which an uptake can be considered significantly positive for a pathological finding. A dissociation of benign findings, such as adrenal adenoma with a moderate uptake, is not always easy.
The risk of misinterpreting any positive uptake as pathological may lead to an increased rate of false-positive findings. On the other hand, smaller or extra-adrenal findings bear a great challenge, precisely because they can be easily overlooked, particularly if planar imaging is performed or if the findings are located next to physiological accumulations or organs.
It should be noted at least that larger adrenal masses, already considered to be suspicious in other imaging tools such as MRI because of their density, might show no or very low mIBG uptake. In such cases an adrenal carcinoma cannot be ruled out. An operation with histological confirmation is so far the only opportunity to definitively assure diagnosis.
Some hints to reduce false-positive or false-negative results:
Correct patient selection criteria are required, which means positive biochemical testing and/or evaluation of suspicious masses of the adrenal in other imaging modalities.
In utilization of SPECT/CT, the particular strengths of mIBG SPECT/CT are detection of local recurrence, small extra-adrenal phaeochromocytomas and multifocal tumours (Fig. 6.6).
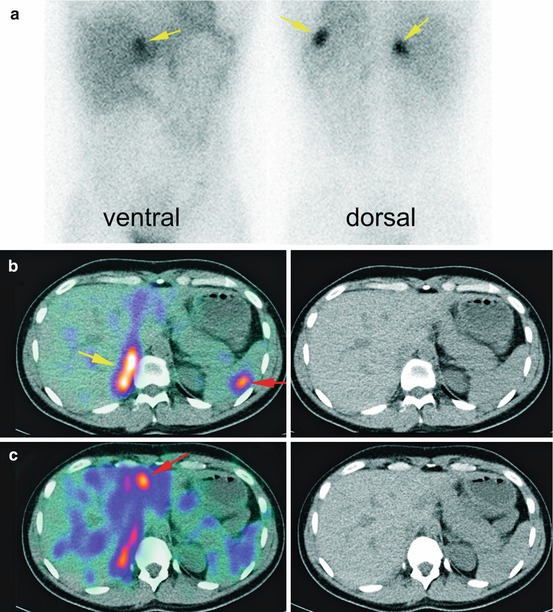
Fig. 6.6
Planar images of a patient with suspected recurrence of phaeochromocytoma showed three suspected abdominal uptake (a, yellow arrows). SPECT/CT revealed the exact anatomical localization of these uptakes (b, c) local recurrence (yellow arrow), a spleen metastasis and a liver metastases (red arrows)
6.19 mIBG SPECT/CT in Medullary Thyroid Carcinoma
Medullary thyroid carcinoma (MTC) is a quite rare thyroid tumour which occurs in 5–10 % of all thyroid carcinomas and arises from thyroid parafollicular C-cells, which are embedded in the thyroid but do not belong to the thyrocytes. It releases calcitonin, CEA and several other substances, such as chromogranin, serotonin, somatostatin and gastrin-releasing peptide.
A sensitive and specific marker for the occurrence of MTC is calcitonin, which is produced by the C-cells. An elevated calcitonin level (hypercalcitoninaemia) reflects a disorder, a reactive stimulation of the C-cells or impaired/disturbed degradation for calcitonin. There is a wide range of differential diagnoses for increased calcitonin levels, such as alcohol consumption, intake of various drugs (calcium, proton pump inhibitor, medication with calcitonin), hypercalcaemia and adrenal insufficiency. Also, a severe bacterial infection, a serious illness or a paraneoplastic syndrome can also cause a hypercalcitoninaemia. If reasons such as these are excluded, only C-cell hyperplasia (CCH, C-cell hyperplasia) and MTC remain as the two most frequent diagnoses. It should be noted that C-cell hyperplasia is associated specifically with males, in combination with autoimmune thyroiditis or hyperparathyroidism-associated hypergastrinaemia.
The reference values for basal calcitonin are higher in men than in women. Hypercalcitoninaemia should be confirmed in a second measurement. The interpretation of an elevated calcitonin level between10 and 100 pg/ml is ambiguous; only a basal calcitonin level >100 pg/ml is almost always accompanies an MTC. If available, for further diagnosis, a pentagastrin test should be carried out. If pentagastrin stimulation is not available, stimulation can also be performed with calcium, noting that after calcium stimulation, false-positive findings appear to be more common in female patients and patients with thyroiditis and thyroid neoplasia, other than MTC [54]. 131/123I-mIBG uptake in MTC appears via the same molecular mechanisms, just as in other neuroendocrine tumours such as phaeochromocytoma [7].
6.20 Imaging Procedures
Cross-sectional imaging studies employing neck, chest and three-phase abdominal CT scans or contrast-enhanced MRIs are recommended to rule out distant metastases, especially when suspicious lymph nodes are identified or when calcitonin levels are >400 pg/ml [55, 56].
There are also several radiotracers to mention, such as 99mTc-(V)-DMSA, 123I-mIBG and 111In-labelled somatostatin analogue and the PET pharmaceuticals 18F-FDG, 68Ga-DOTATOC and 18F-DOPA [57].
The use of 123I-mIBG in MTC is not the gold standard procedure because of its low sensitivity, which is said to be approximately 35 % especially for familial/MEN-associated MTC. For sporadic MTC, data are superior. The specificity of mIBG is quite high and is approximately 95 % [57–61].
In summary, the role of 131/123I-mIBG in the diagnostic evaluation of MTC is limited, but it still serves as an evaluation method for tentative therapy with 131I-mIBG [7].
Moreover, a combined diagnosis of 131/123I-mIBG scan and somatostatin receptor scintigraphy (SRS) increases the sensitivity up to 100 % and seems to be the best practice to choose the most effective radiopharmaceutical with regard to therapy options [57].
The use of 131/123I-mIBG SPECT/CT can serve as a follow-up to individual metastases and their responses to therapy, as SPECT/CT, more than planar imaging, simplifies localization and evaluation of the tumour burden.
References
1.
Nakajo M, Shapiro B, Copp J, Kalff V, Gross MD, Sisson JC, et al. The normal and abnormal distribution of the adrenomedullary imaging agent m-[I-131]iodobenzylguanidine (I-131 MIBG) in man: evaluation by scintigraphy. J Nucl Med. 1983;24:672–82.PubMed
2.
Bombardieri E, Giammarile F, Aktolun C, Baum RP, Bischof Delaloye A, Maffioli L, et al. 131I/123I-metaiodobenzylguanidine (mIBG) scintigraphy: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging. 2010;37:2436–46. doi:10.1007/s00259-010-1545-7.PubMed




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree