Fig. 5.1
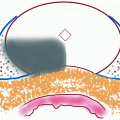
Diagram illustrating that the likely origin of metastases is from one clone within the prostate, which evidence points to it being within the index lesion—the largest lesion with the highest grade. A prostate can have more than one index lesion which fulfills a threshold volume or grade that gives rise to metastases
Contrary to the preceding studies, Ruijter et al. [10] found that a quarter of the tumors invading the capsule in multifocal disease were not the index lesion. Tumors need not acquire large volume before they become locally invasive. Even small foci, as small as 0.2 ml in size, may show a significant release of tumor cells into the bloodstream [25] and can give rise to lymph-node metastasis [26]. In a series of 239 patients with tumor volume less than 0.5 ml, Kikuchi et al. [27] found that 43 were poorly differentiated, 11 had extracapsular extension, 6 had positive surgical margins, 2 had positive lymph nodes, and 7 experienced progression within 5 years. Greene et al. [28] evaluated the DNA ploidy status, an independent prognostic factor for localized prostate cancer. Of 141 separate cancers in 68 patients, they observed that 15% of those 0.01–0.1 ml and 31% of those 0.11–1.0 ml in volume were non-diploid. Thus, tumor volume in itself might not adequately describe the biological potential of prostate cancer.
While a small secondary lesion might be clinically insignificant, multifocality per se may constitute a surrogate prognostic factor independent of the volume of the index and secondary tumors. The presence of multiple tumors within the same gland may reflect genetic instability that allows for accelerated disease progression.37 Magi-Galluzzi et al. [29] examined the pathologic features of unifocal disease in 17 prostate glands obtained at radical prostatectomy. All cancers were identified in the peripheral zone, predominantly involved one lobe, and were associated with unifocal rather than multifocal high-grade PIN. The authors concluded that single clone disease may be biologically different and less aggressive than multifocal disease. Similarly, Djavan et al. [30] observed that multifocal prostate cancers were associated with poorly differentiated tumors with higher stage and recurrence rate than unifocal prostate cancer.
The prognosis for patients with prostatic cancer might be influenced by tumor focality. From the above-mentioned studies, it appears that patients with unifocal disease may follow a more indolent clinical course than those harboring multifocal disease. This hypothesis, however, has recently been questioned by Rice et al. [31] who showed that patients with single focus adenocarcinoma had statistically higher rates of positive surgical margins, Gleason score 8–10 disease, and biochemical recurrence than those who had multifocal disease. In an autopsy study of 264 prostates from men who died of diseases other than prostate cancer, another group found no significant difference with respect to total tumor volume, capsular penetration, perineural or vascular invasion of multifocal when compared with unifocal tumors [32].
Advocates of focal therapy argue that ablation of the index lesion alone and close monitoring of the secondary tumors for early signs of progression might be the cornerstone of successful focal treatment. Much of the original evidence supporting use of active surveillance might conceptually be applied to focal therapy. Clinical experience with active surveillance now suggests that there is an estimated risk of metastasis of <1% at 28 years [33–35], and disease-specific mortality is 1% at 8 years while on surveillance. Warlick et al. [36] evaluated whether curability (defined as surgical pathology consistent with a >75% chance of remaining biochemical recurrence free at 10 years after surgery [37]) is lost among patients with small, low-grade prostate cancers in whom delayed curative treatment was required. The authors compared the rates of incurable cancer among patients undergoing delayed curative surgery at a median of 26.5 months after diagnosis to those among patients undergoing immediate curative surgery and who would have been eligible for expectant management. Time between diagnosis and surgery was not associated with the risk of incurable cancer. It seems that patients with small-volume, low-grade cancer monitored with a rigorous protocol for disease progression have the same risk of incurable prostate cancer for at least 2 years after diagnosis as patients who received immediate prostate cancer surgery. Therefore, one could argue that if it is safe to undergo surveillance in some carefully selected men (on the basis of repeat biopsy, PSA kinetics, and findings on digital rectal examination), the same strategy could be applied to secondary tumors after index ablation.
Research by Klotz et al. [38] may have conflicting implications on this assertion. They report the results of an active surveillance approach in 450 patients after a median follow-up of 6.8 years. Although there was no difference in overall survival between patients who remained on surveillance and those who were reclassified and treated radically, half of the men who underwent radical treatment experienced biochemical failure. All prostate cancer-related mortality occurred in men who had been reclassified as higher risk and who were offered radical treatment. However, only one patient in this series who was treated after a relatively prolonged period of observation subsequently experienced progression to metastatic disease and death. Therefore, it is likely that reclassification of disease risk and subsequent radical treatment reflects undersampling of the prostate rather than true progression. This observation highlights the necessity to incorporate innovative imaging tools such as MRI and template biopsy in the selection and monitoring of patients undergoing active surveillance. Recently, two groups have underlined the low malignant potential for Gleason grade 6 disease. Eggener et al. [39] demonstrated that of 9,775 men who had only Gleason 6 low risk disease in radical whole-mount prostatectomy specimens, only 3 died of prostate cancer in a 15-year period. Such a small number cannot be fully accounted for by the success of surgery itself. Another group showed similar findings with biochemical recurrence in a smaller cohort of men [40].
Lin et al. found that in primary prostate tumors metastatic potential can be confined to a minority of cancer cells using pieces of tissue from different foci of a patient’s primary prostate tumor that were grafted into subrenal capsules of NOD-SCID mice and transplantable tumor sublines were established by serial passage. The metastatic ability of each subline was tested via orthotopic grafting into mice [41]. Further studies have shown that metastatic tissue in lymph nodes has the same TMPRSS–ERG gene fusion matching only lesions that are the index or those that are high Gleason grade even if secondary lesions [42, 43]. Liu et al. [44] found that prostate cancer metastatic deposits in individual men have a monoclonal origin in most cases through an elegant autopsy study. Although the question of whether the metastasis clone was derived from and situated in the index lesion was not addressed by the authors, it seems likely that the precursor cells are found in the index lesion considering the pathological characteristics. Indeed, we have previously shown that histopathological features of poor prognosis in the prostate such as tumor volume, Gleason score, and pathological stage are almost invariably determined by the index lesion [45]. The monoclonal origin of metastatic prostate cancer has given fresh impetus to the idea of focal therapy for the majority of men who have multifocal, bilateral disease in which only the clinically significant lesion might be ablated [46]. Obviously, the concept of cancer that is “biologically unifocal” appears attractive when considering focal therapy. However, the previously mentioned studies underlying the potential clinical significance of satellite lesions suggest that even small secondary foci may require treatment. Therefore, advocacy of subtotal treatment with the knowledge that smaller secondary foci remain untreated continues to be controversial at present.
Although the goal of focal therapy for prostate cancer is to selectively ablate known disease, it should be remembered that a field effect might occur; that is neoplastic or paraneoplastic cells exist in the apparently histologically normal field adjacent to the tumor. Genetic alteration and molecular changes have been found in normal appearing prostate tissue adjacent to tumor, consistent with the possibility of a field effect. This finding seems to be dependent on the distance from the cancer focus but not in a linear fashion [47–49]. Although these studies theoretically suggest that the concept of focal ablation of the tumor area requires cautious robust study, current evidence suggests the field effect occurs in the immediate vicinity of the cancer focus and therefore an adequate margin around the cancer could be incorporated into treatment together with the lesion [50]. The field effect concept is accepted in the treatment of other solid organ cancers with the use of adjuvant therapy to the remaining tissue (low-dose radiotherapy after wide local excision in breast cancer) and surveillance on a regular basis (mammography for breast cancer, CT for kidney cancer). Surveillance for untreated prostate cancer has been developed formally within active surveillance cohort studies and similar principles may apply to untreated tissue harboring no clinically significant cancer.
Funding Sources and Acknowledgements
H.U. Ahmed and M. Emberton receive funding from the Medical Research Council, UK NIHR-HTA, Pelican Cancer Foundation, Prostate Action, Prostate Cancer Research Centre and St Peters Trust for work on focal therapy and imaging of prostate cancer. M. Emberton has also been funded in part by the UCH/UCL NIHR Comprehensive Biomedical Research Centre.
References
1.
2.
3.
4.
5.
6.
Noguchi M, Stamey TA, McNeal JE, Yemoto CE. Assessment of morphometric measurements of prostate carcinoma volume. Cancer. 2000;89(5):1056–64.PubMed




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree