Section II
CARDIOLOGY
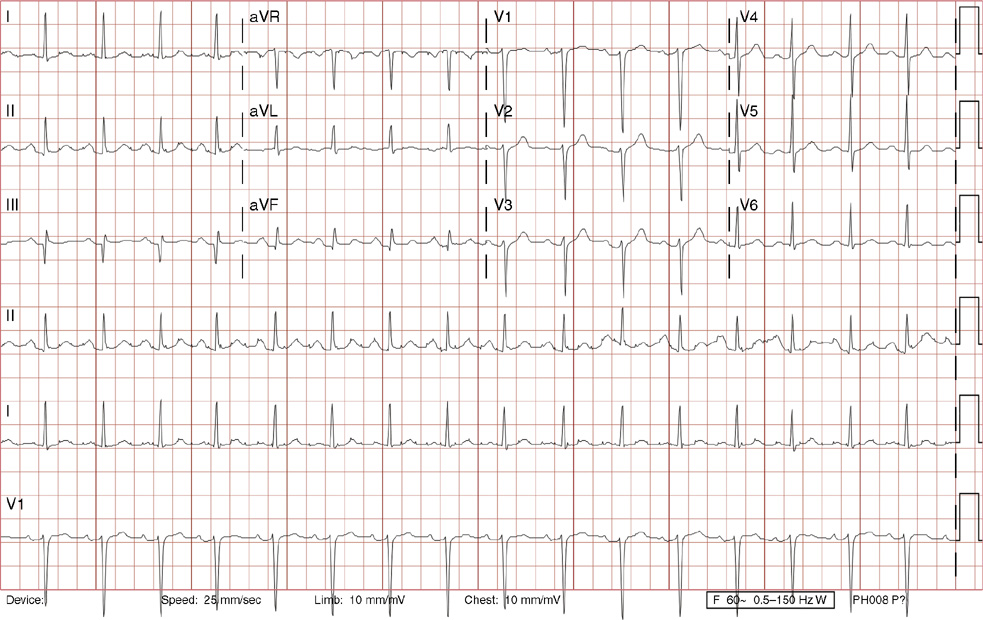
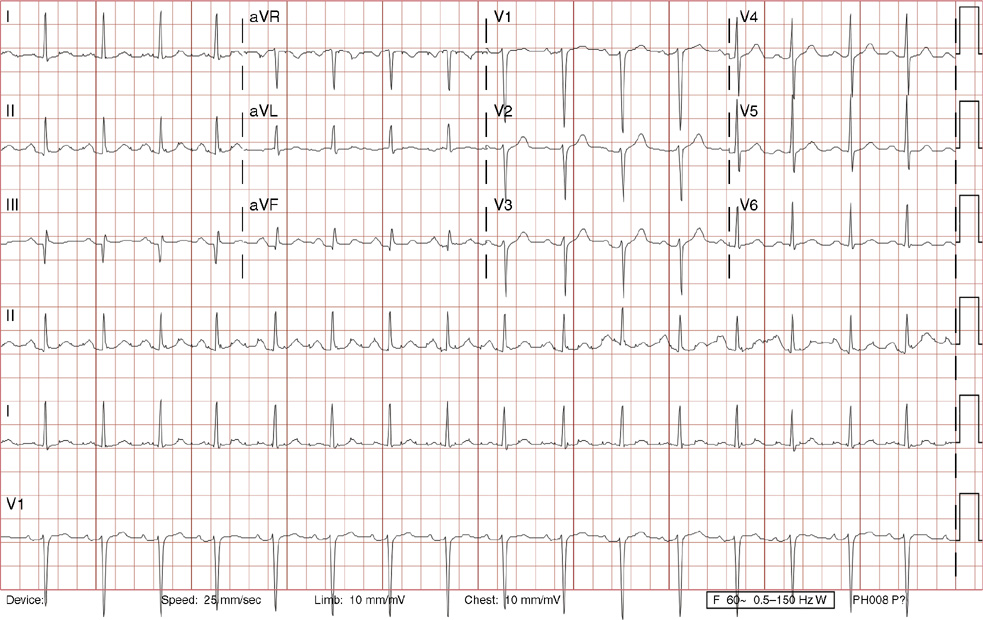
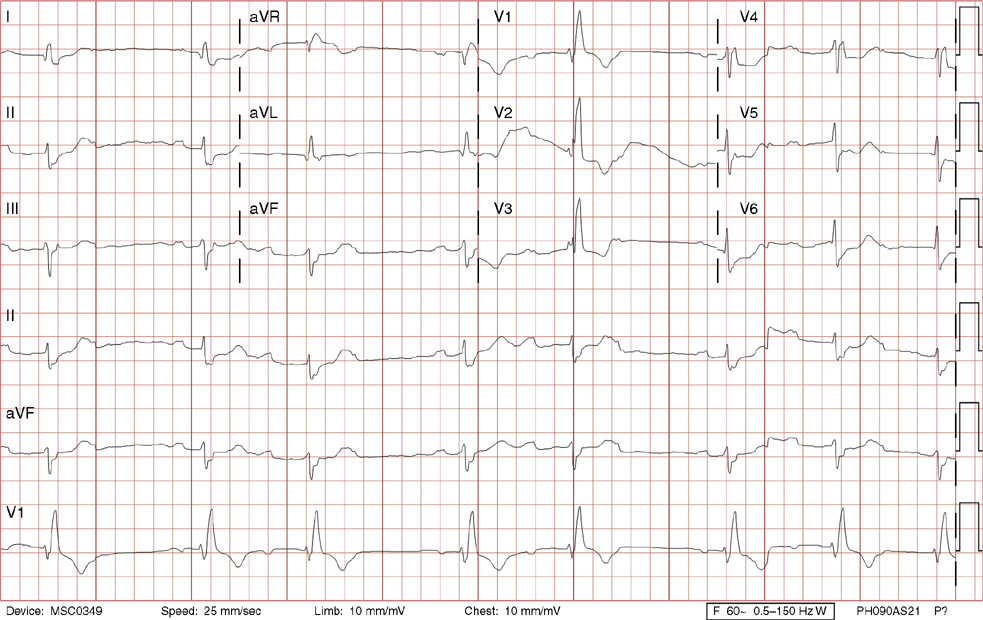
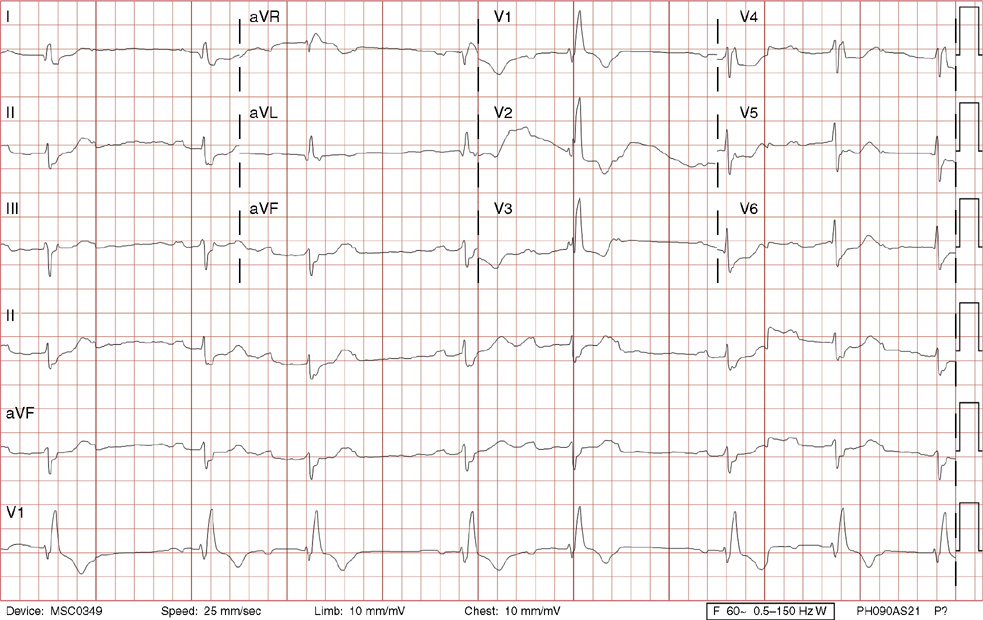
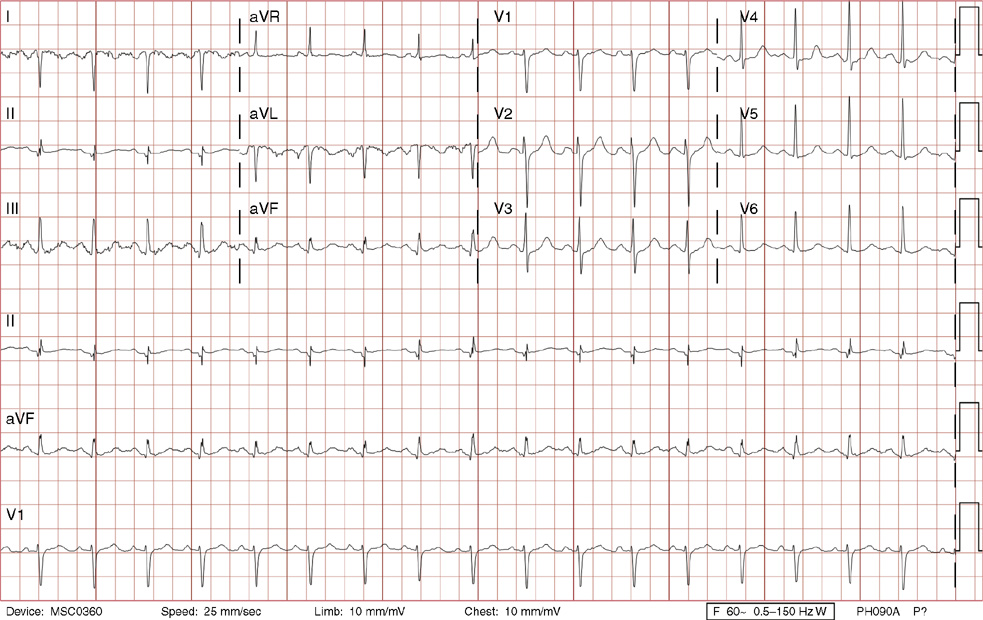
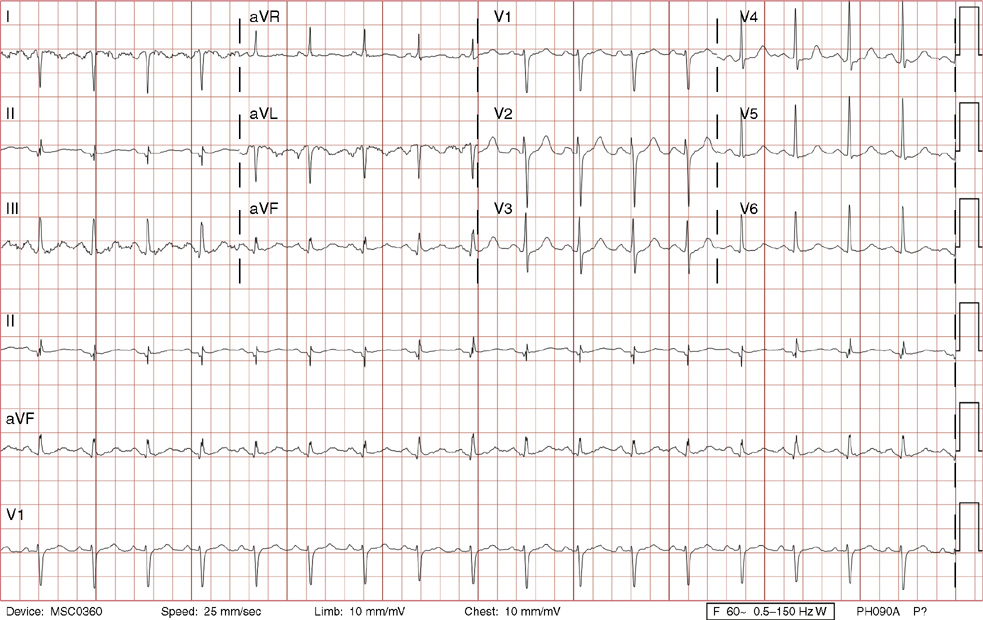
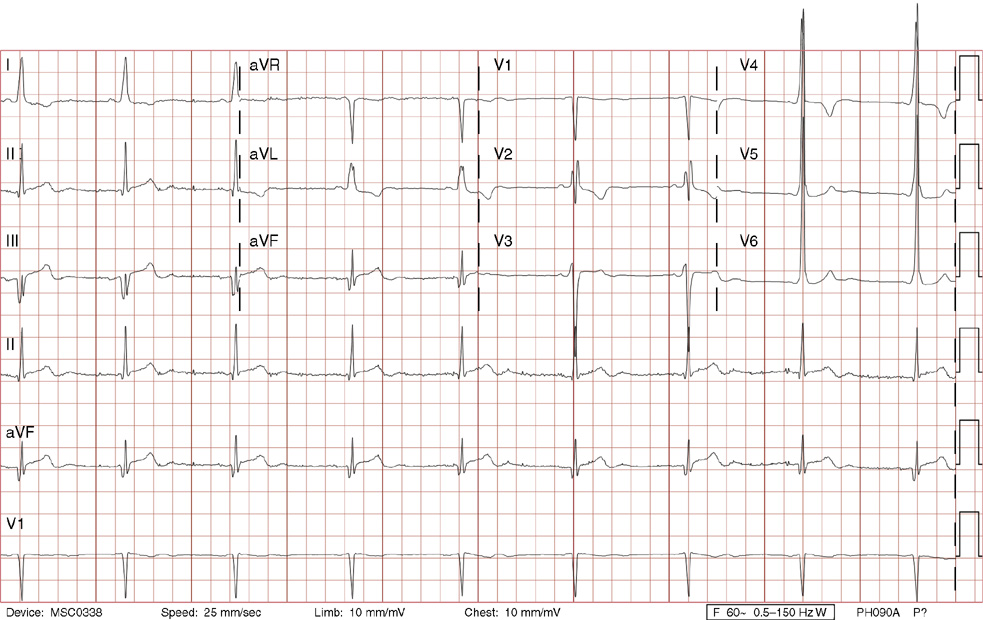
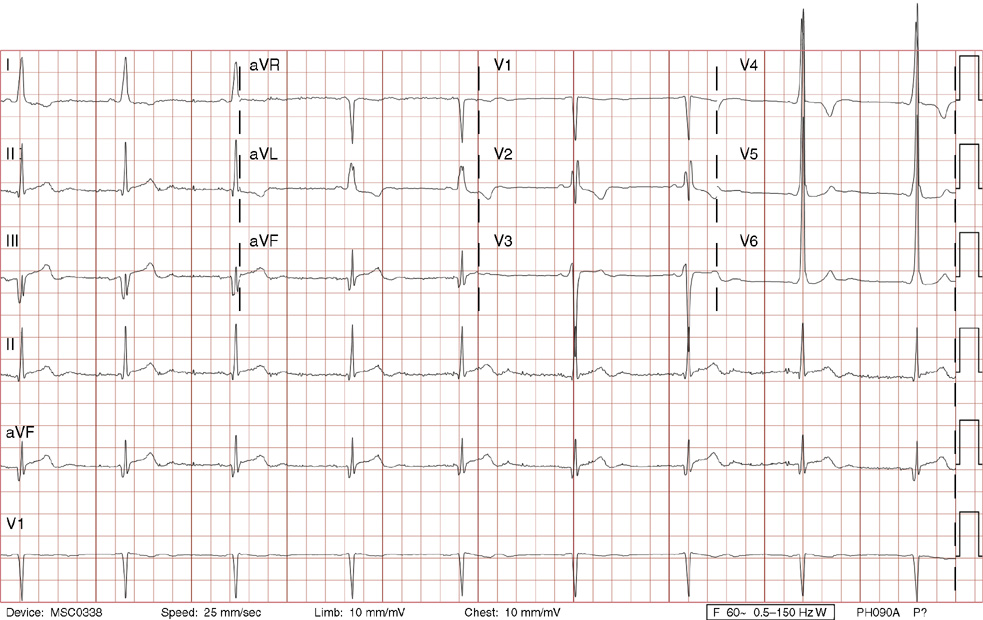
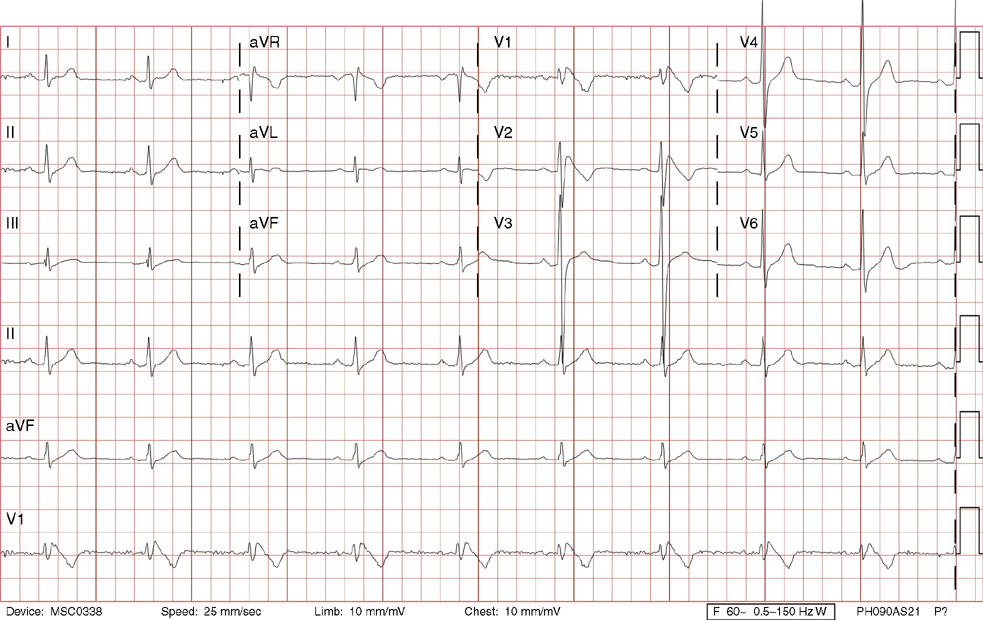
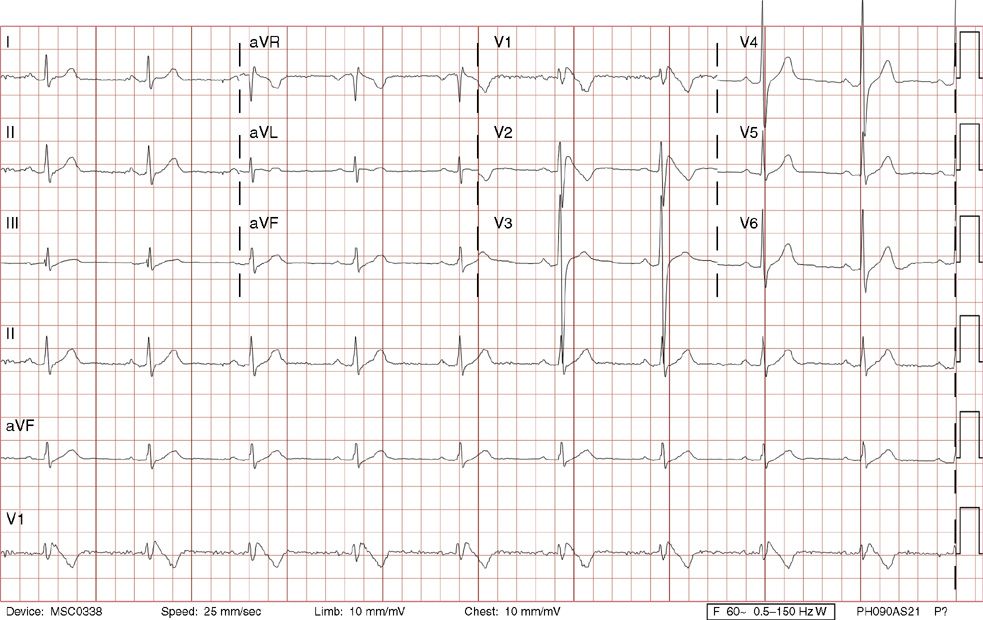
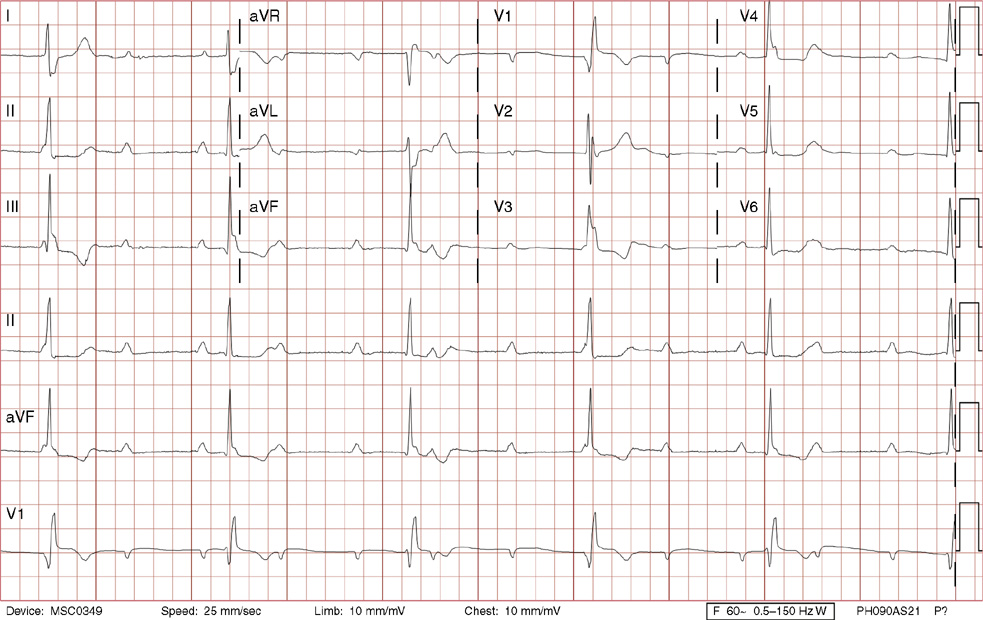
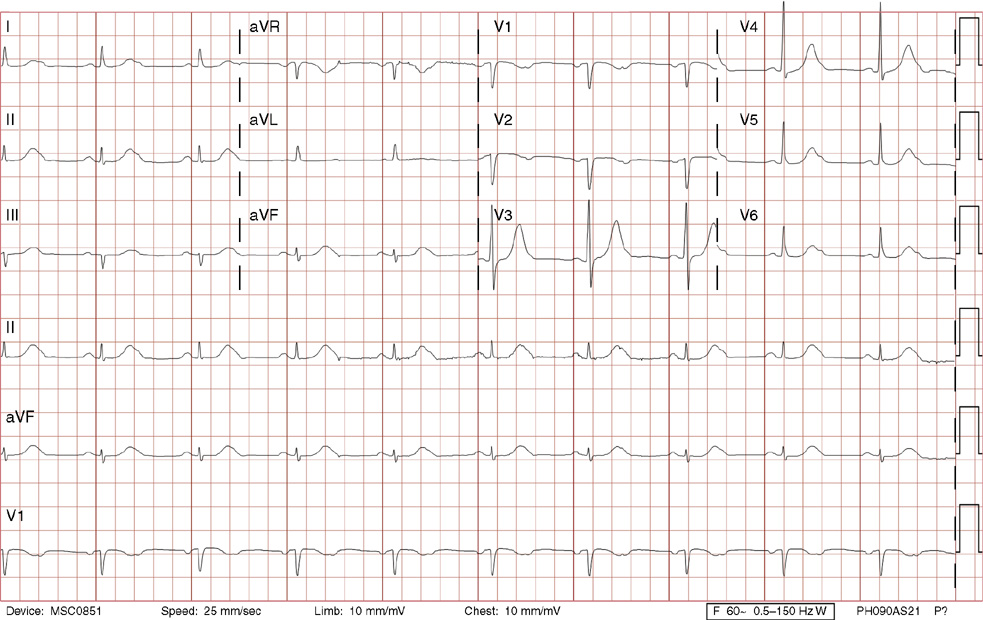
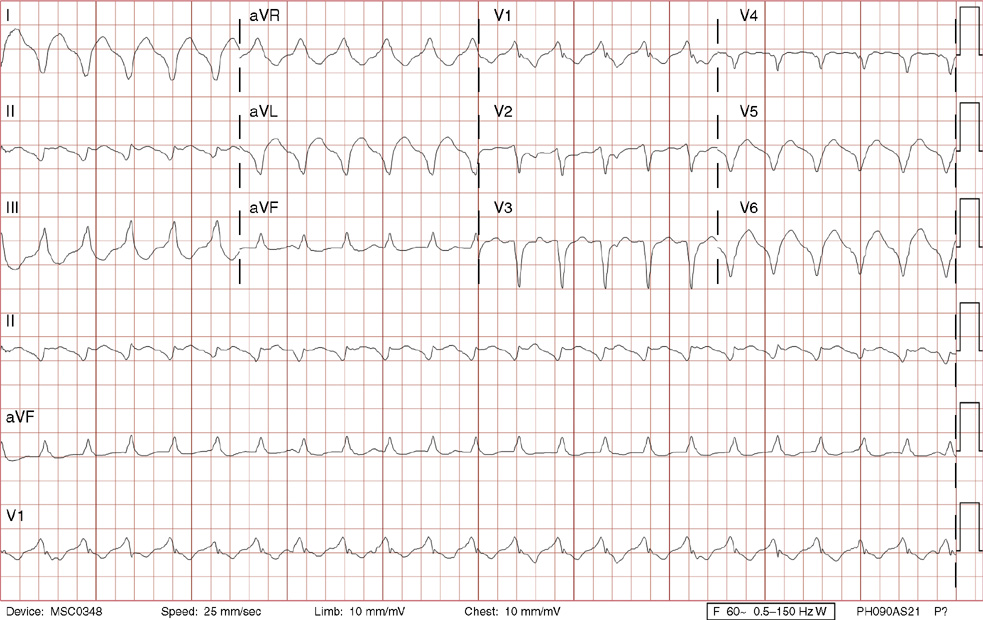
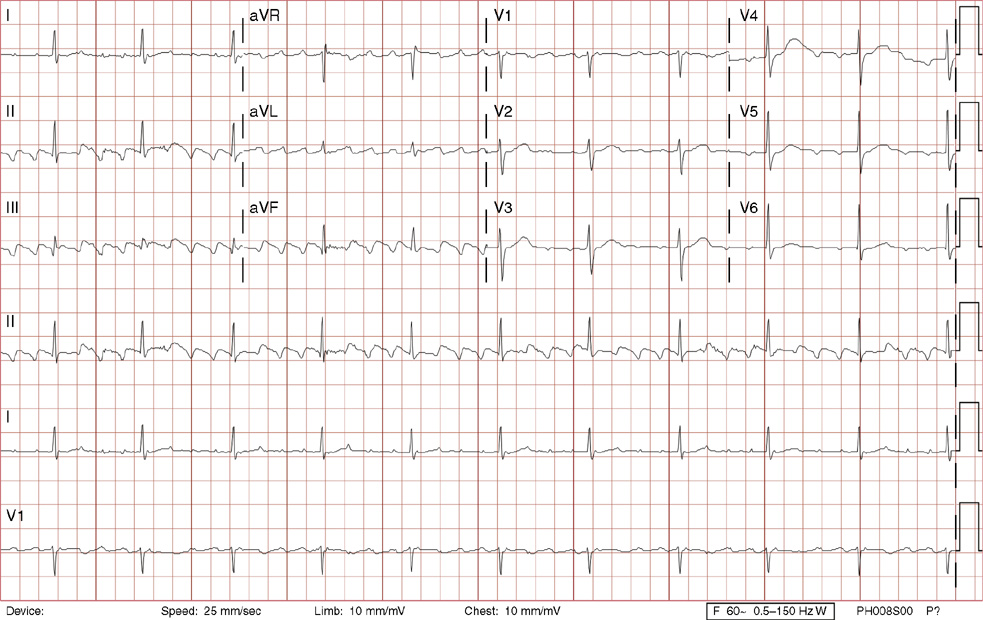
1. A 30-year-old woman presents to her primary care physician with complaints of occasional palpitations occurring over the last 10 years. She currently is asymptomatic and has no significant past medical, family, or social history. The electrocardiogram (ECG) shows:
A. Atrial tachycardia with inferior infarct, age indeterminate
C. Atrioventricular reentrant tachycardia
D. Sinus tachycardia with inferior infarct, age indeterminate
E. Sinus tachycardia with borderline poor R wave progression
1. The answer is E: Sinus tachycardia with borderline poor R wave progression. Poor R wave progression is defined as the absence of transition from a negative to positive QRS complex in the precordial leads by lead V4. Borderline poor R wave progression can be defined as transition appropriately occurring by lead V4, but inappropriately delayed positive voltage transition from V1 to V3, as in this case. Poor R wave progression can be found in patients with anterior myocardial infarction, left ventricular hypertrophy, and in normal individuals. Sometimes it occurs due to lead misplacement, which is most likely the case in this patient given the clinical history. In fact, this patient’s echocardiogram revealed normal wall motion and overall function.
A, B. This is not atrial tachycardia, because the P waves have an axis consistent with a sinus node origin in the high right atrium (positive P waves in lead II). Rarely an atrial tachycardia could be coming from near the sinus node and be indistinguishable from sinus tachycardia. In this situation, seeing the onset or offset of the tachycardia would distinguish sinus tachycardia from atrial tachycardia—sinus tachycardia begins and ends slowly, whereas atrial tachycardia begins and ends suddenly.
C. When there are narrow QRS complexes, atrioventricular reentrant tachycardia (AVRT) would have retrograde (inverted in lead II) P waves with a different axis. AVRT involves conduction through an accessory bypass tract as part of the reentry circuit.
D. This ECG is not consistent with an inferior infarct. Pathologic Q waves are defined as having a duration greater than 30 ms (~1 small box) and deeper than 0.1 mV (>1 small box) in two contiguous leads. Though the Q wave in lead III meets part of the criteria, the small Q wave in lead aVF does not. Many patients will have an isolated Q wave in lead III, and this is not pathologic.
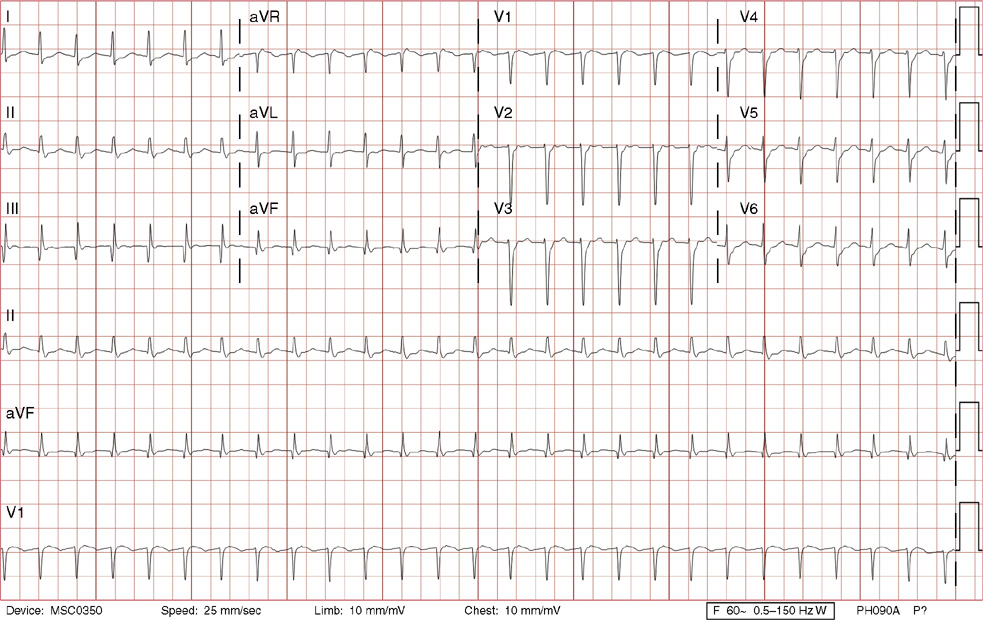
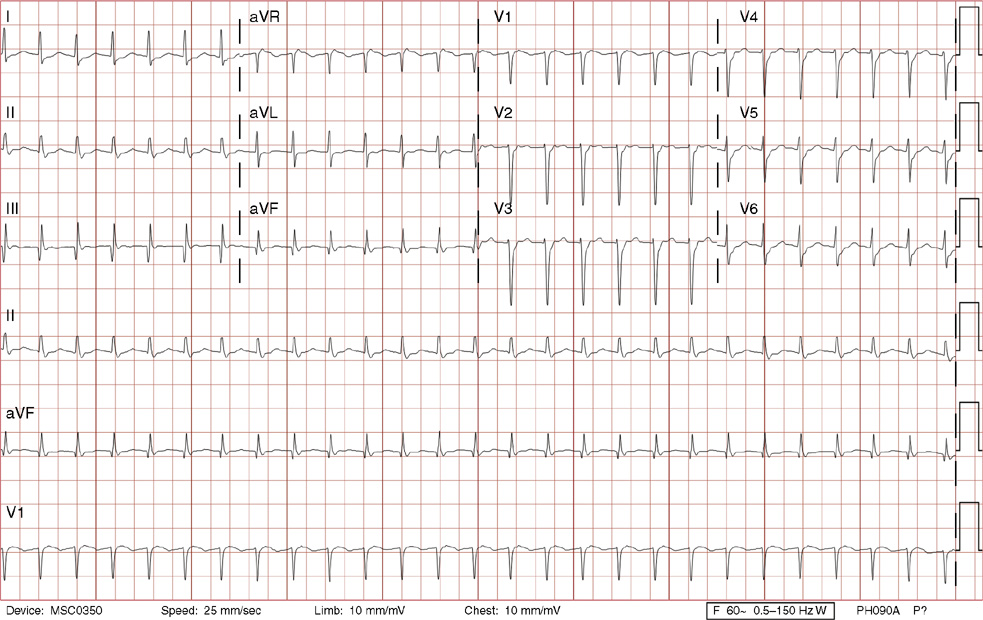
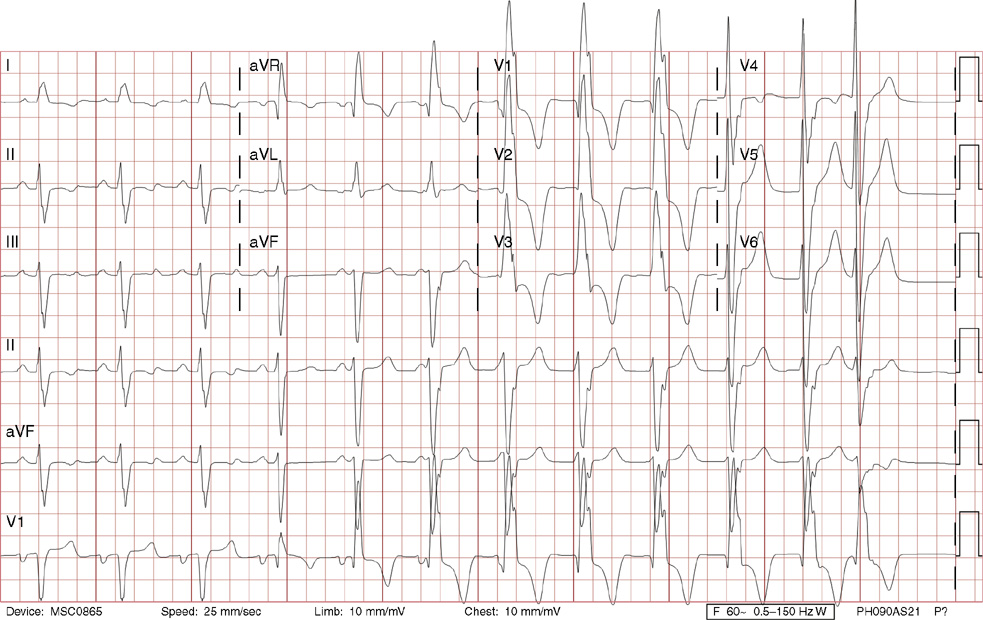
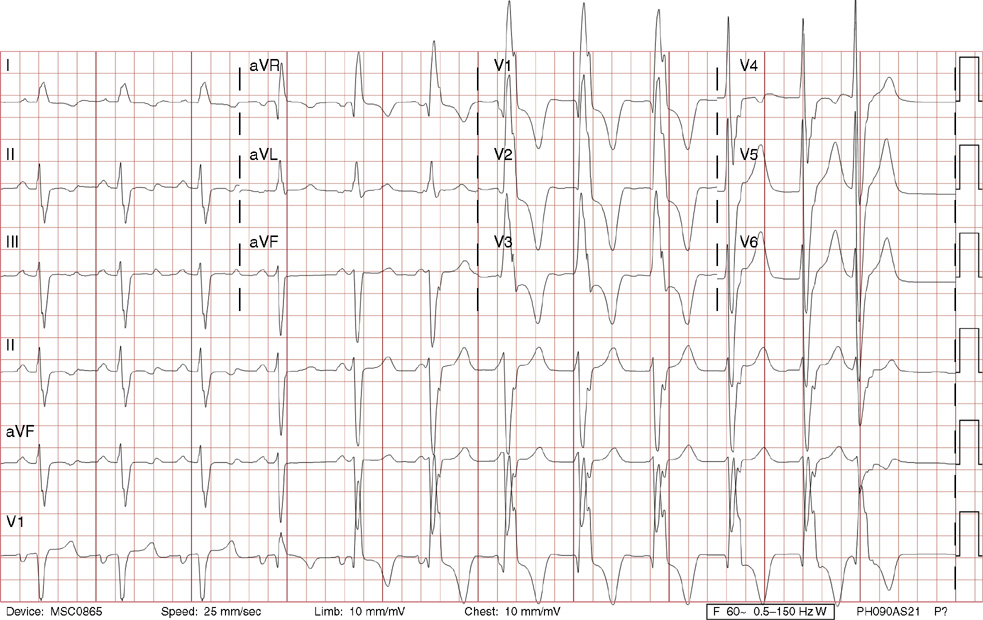
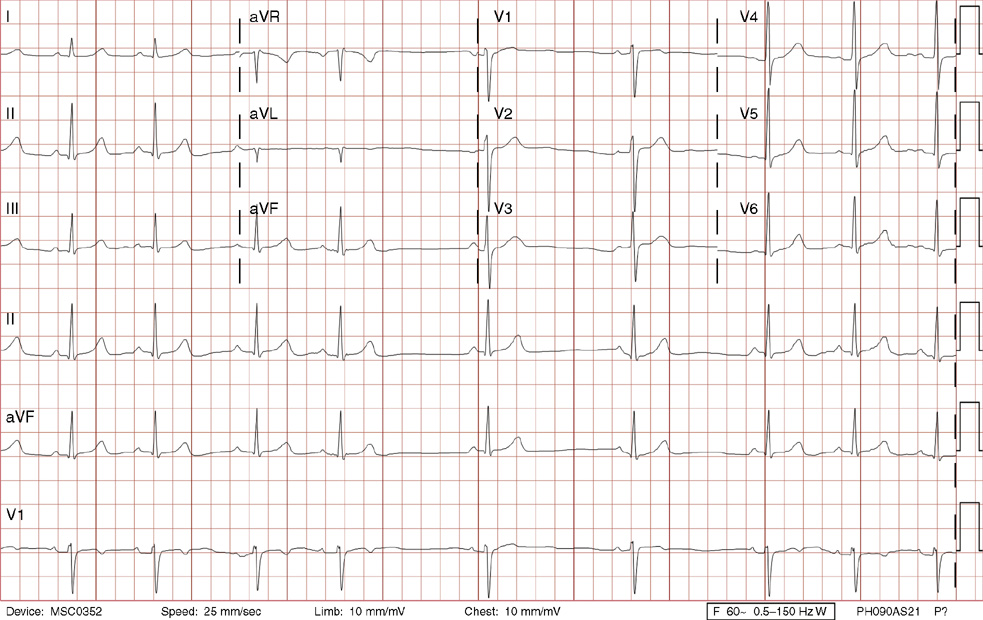
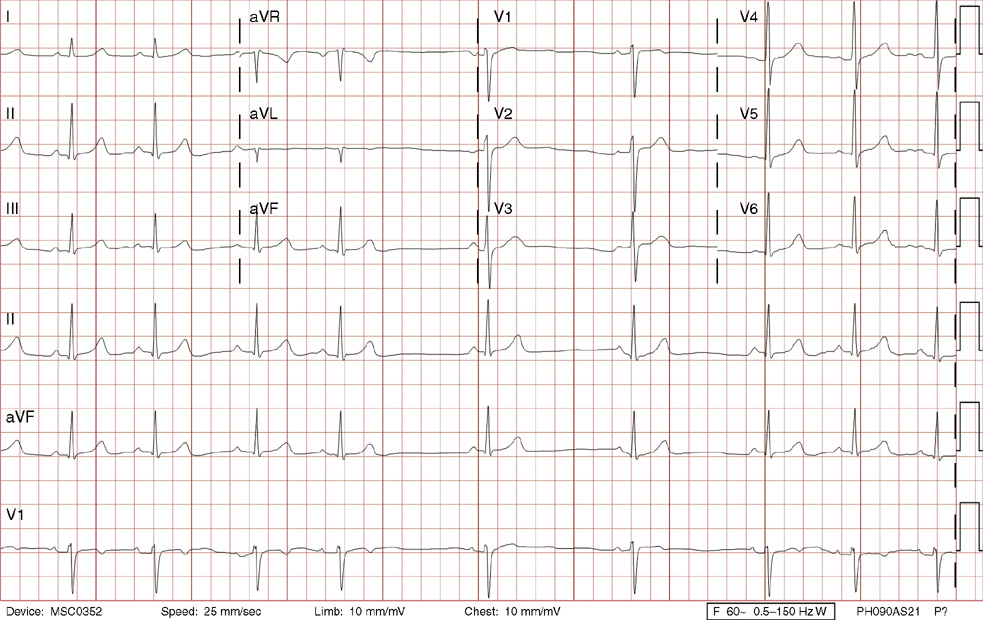
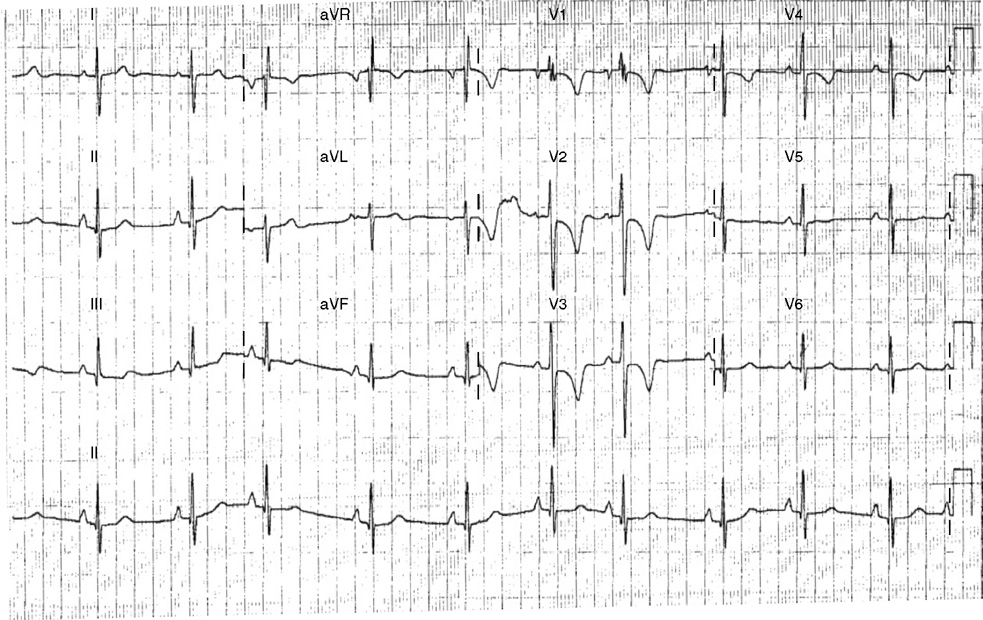
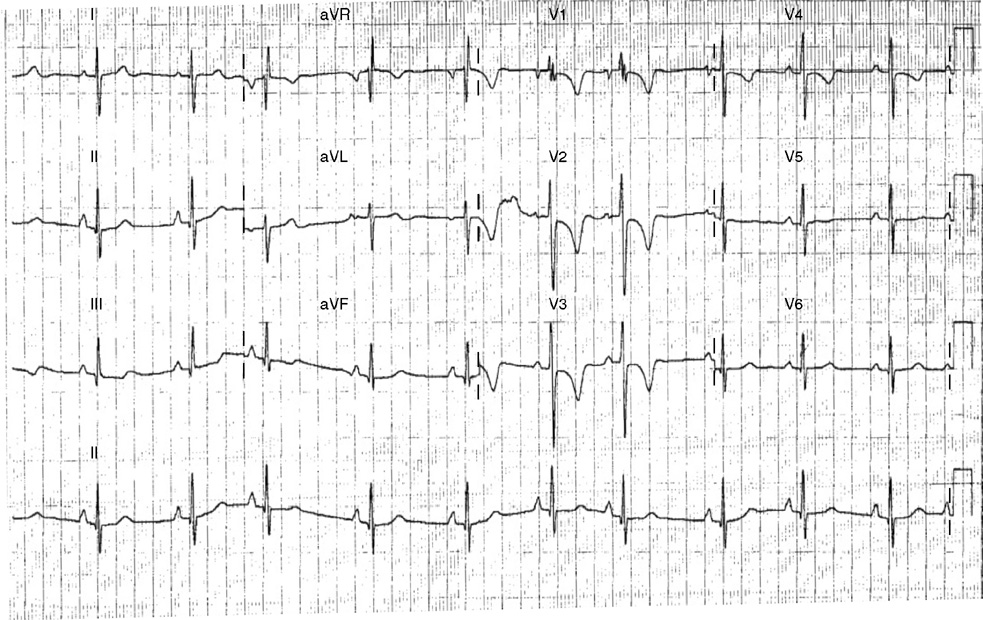
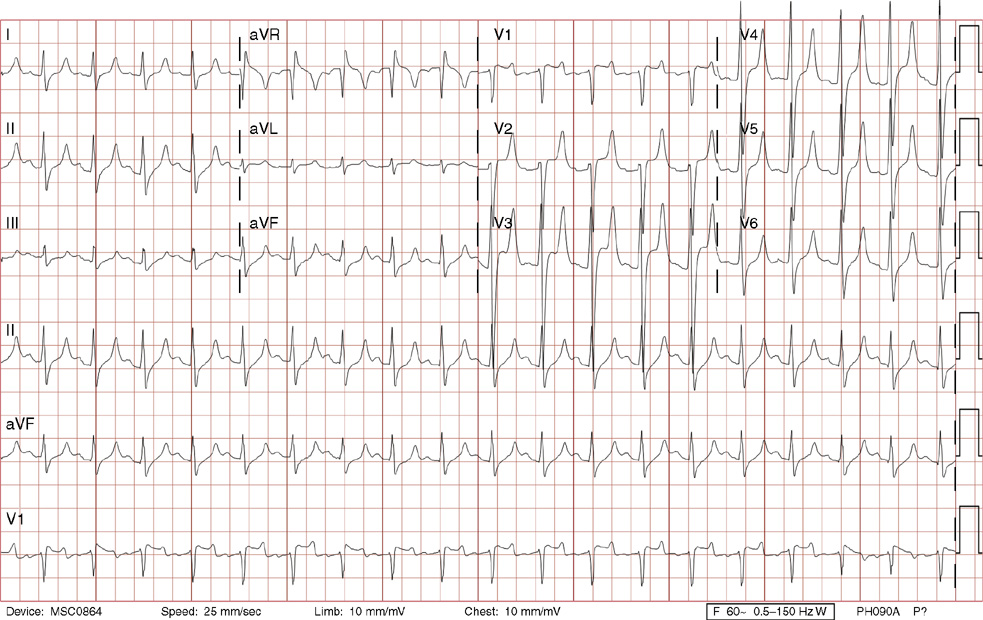
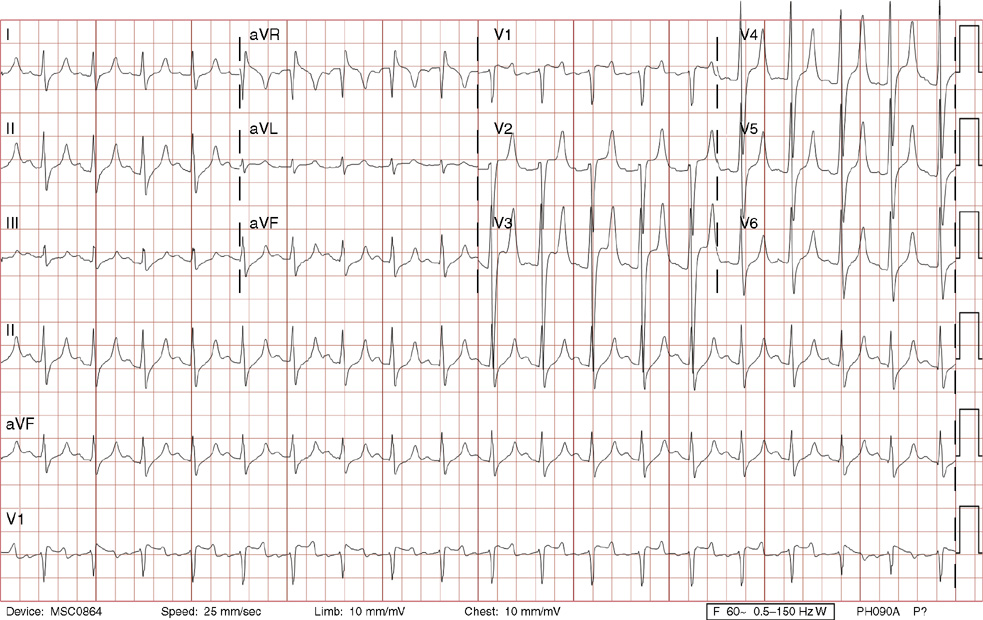
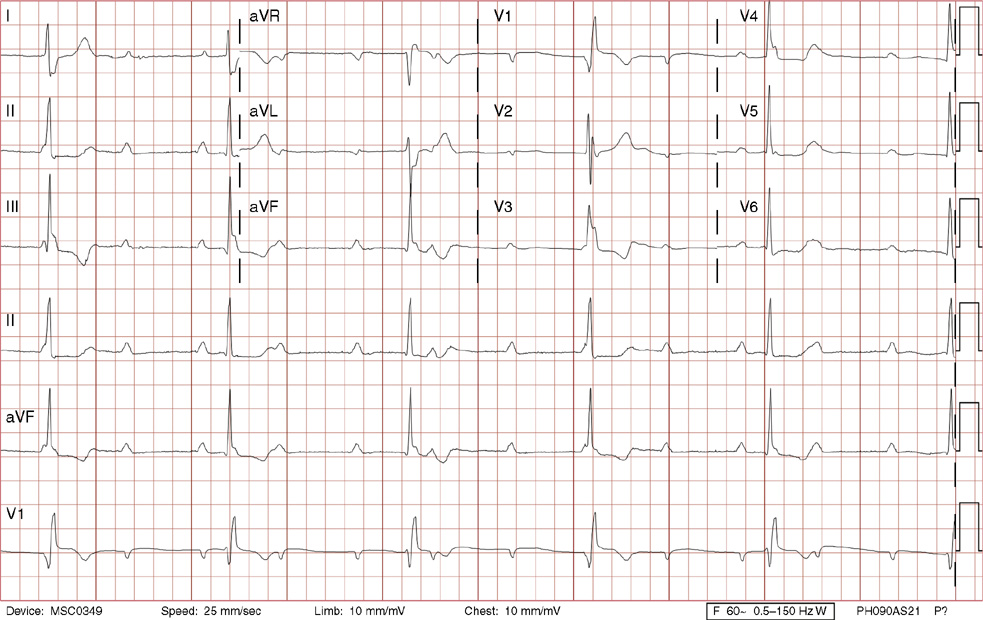
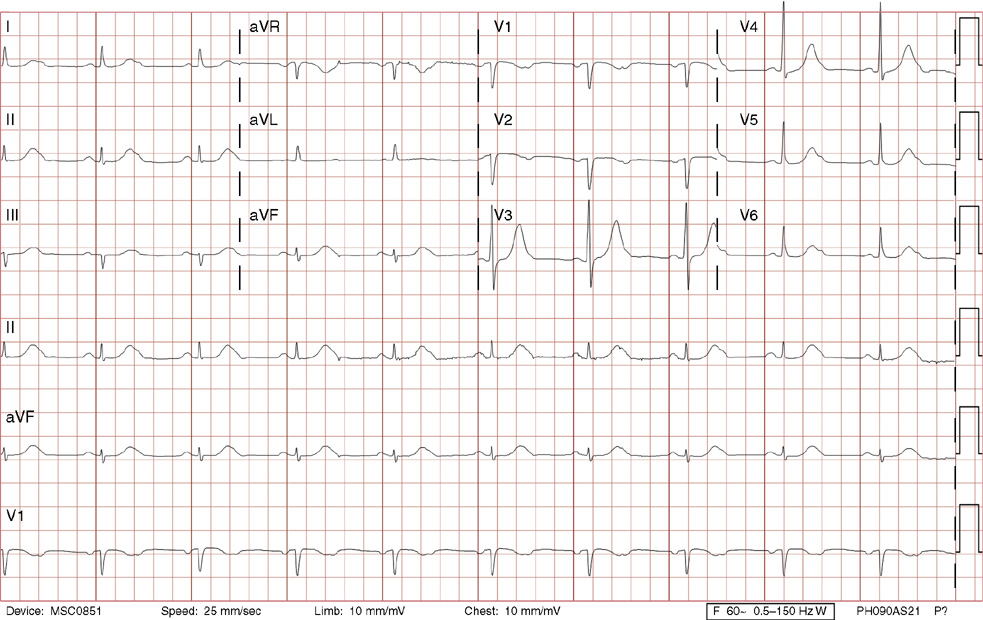
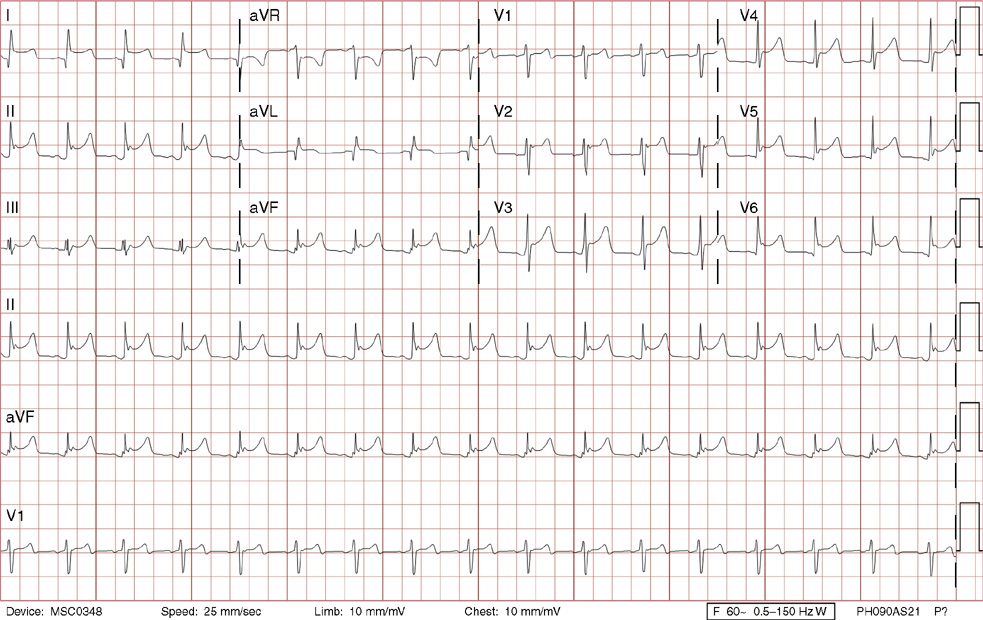
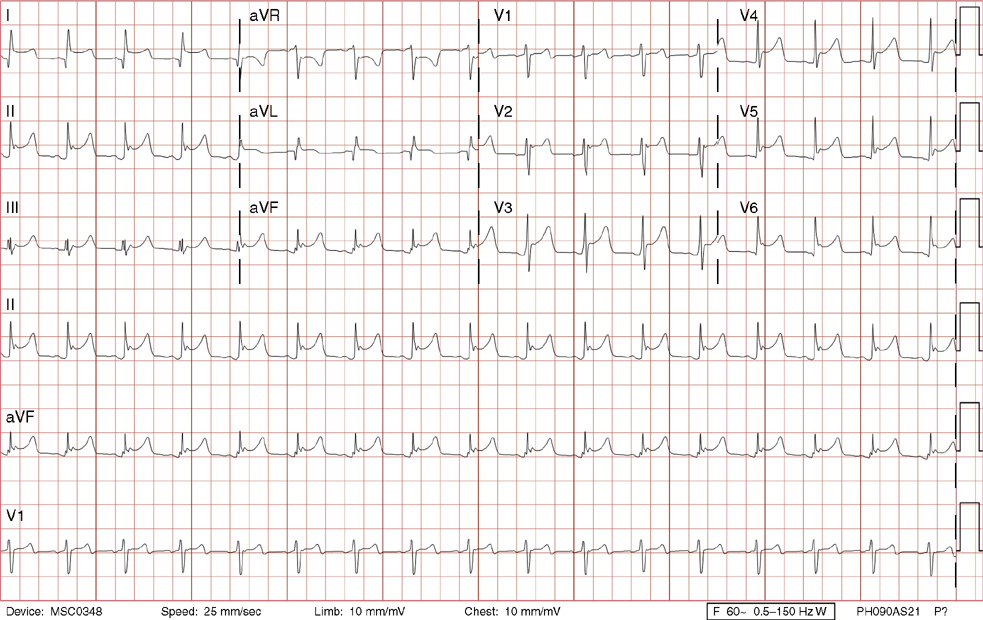
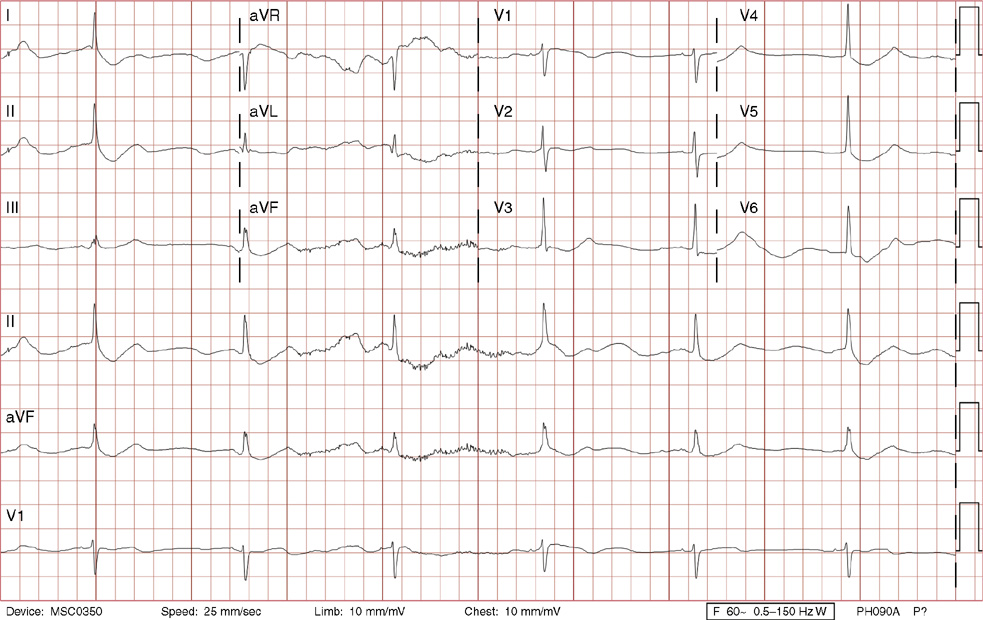
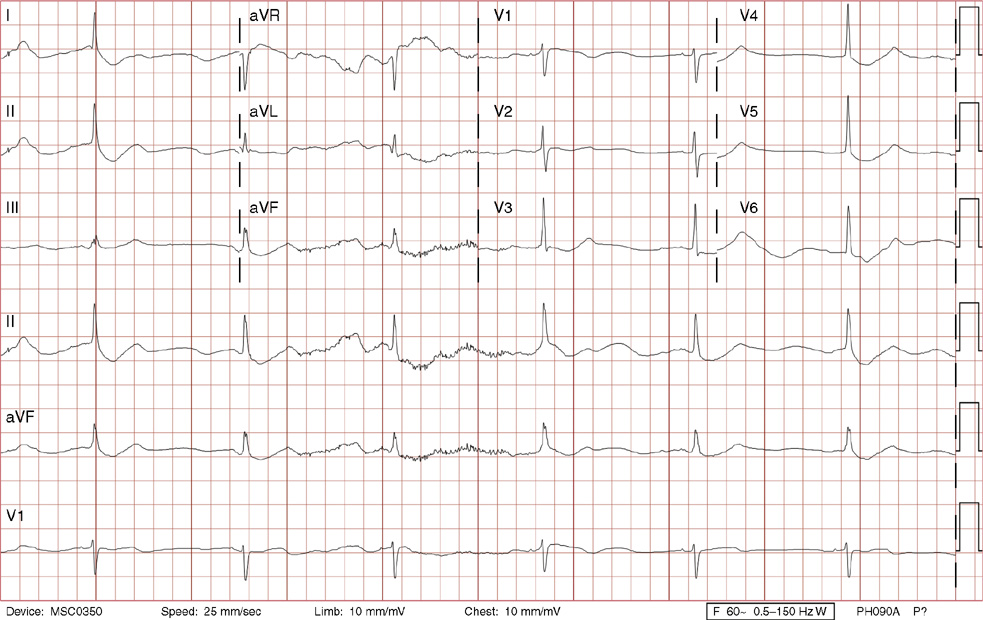
2. The same 30-year-old woman presents to the emergency department 1 month later with the sudden onset of palpitations and mild lightheadedness. She had experienced briefer episodes over the last 10 years, but this episode persisted for 5 minutes. There has been no interval change in her nonsignificant past medical, family, or social history. Her physical exam is normal except for tachycardia. The ECG shows:
B. Ventricular tachycardia (VT)
D. Atrioventricular nodal reentrant tachycardia (AVNRT)
E. Wolff-Parkinson-White syndrome
2. The answer is D: Atrioventricular nodal reentrant tachycardia (AVNRT). This ECG shows AVNRT (also known as atrioventricular nodal reciprocating tachycardia), and poor R wave progression. The Q waves in leads III and aVF are not present in lead II and therefore do not reflect an infarct pattern. AVNRT is a supraventricular tachycardia that commonly occurs in adults with no structural heart disease, most commonly females in the third to fourth decade of life. It presents with palpitations and dizziness, sometimes with dyspnea and chest pain, and rarely syncope. Rates are typically between 140-250 bpm. AVNRT is a reentrant arrhythmia involving the AV node and perinodal tissue with anterograde conduction in a slow pathway and retrograde up a fast pathway, causing near-simultaneous activation of the atria and ventricles. As a result, there may be a inverted P wave noted, most commonly immediately following the QRS complex in the form of a pseudo-S wave, as seen in this tracing in the inferior and left precordial leads (though in some cases this P wave will be buried in the QRS complex or, less commonly, immediately before the QRS). It is helpful to have a baseline ECG for comparison, at which point the retrograde P waves become clearly evident (see question 1, which depicts this patient’s baseline ECG which lacks the retrograde P waves).
A. This ECG is not sinus tachycardia because of the lack of upright P waves in lead II preceding the QRS complex.
B. The QRS complexes are narrow and therefore notVT.
C. The rate of about 150 should prompt the thought of atrial flutter with 2:1 conduction, but there are no flutter waves present.
E. Wolff-Parkinson-White syndrome is the combination of preexcitation (exhibited by a short PR interval and a delta wave due to the faster conduction over the accessory pathway fusing with normal conduction through the AV node), and arrhythmias such as paroxysmal atrioventricular reentrant tachycardia (also known as atrioventricular reciprocating tachycardia). This case lacks a delta wave on the baseline ECG seen in question 1.
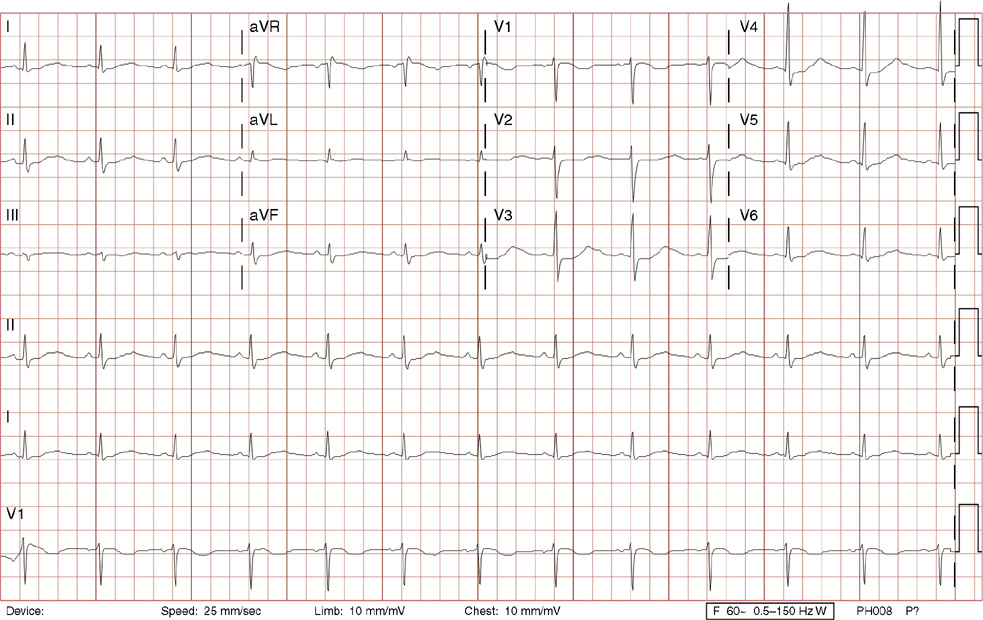
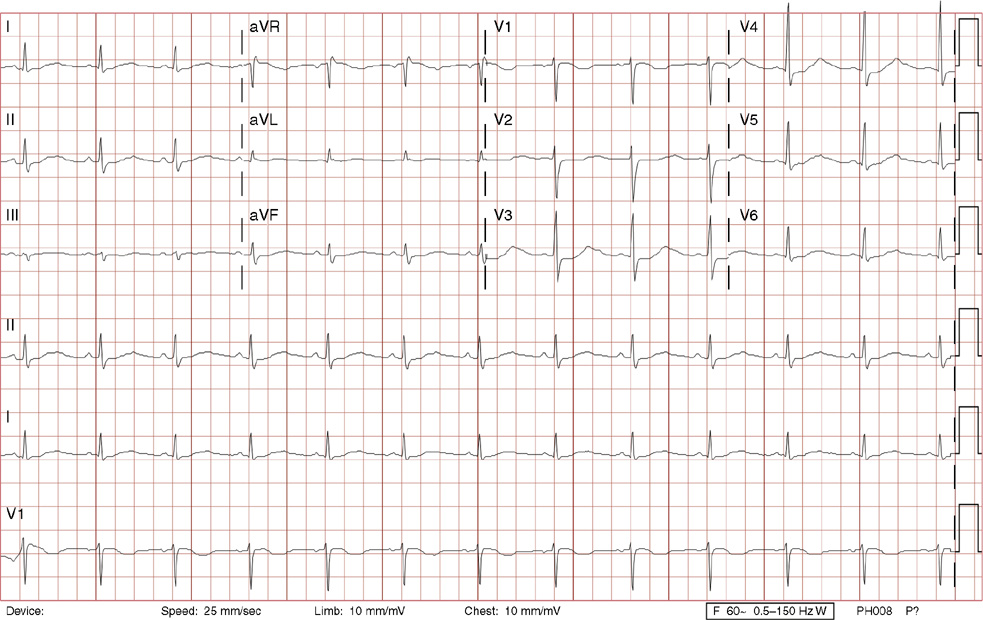
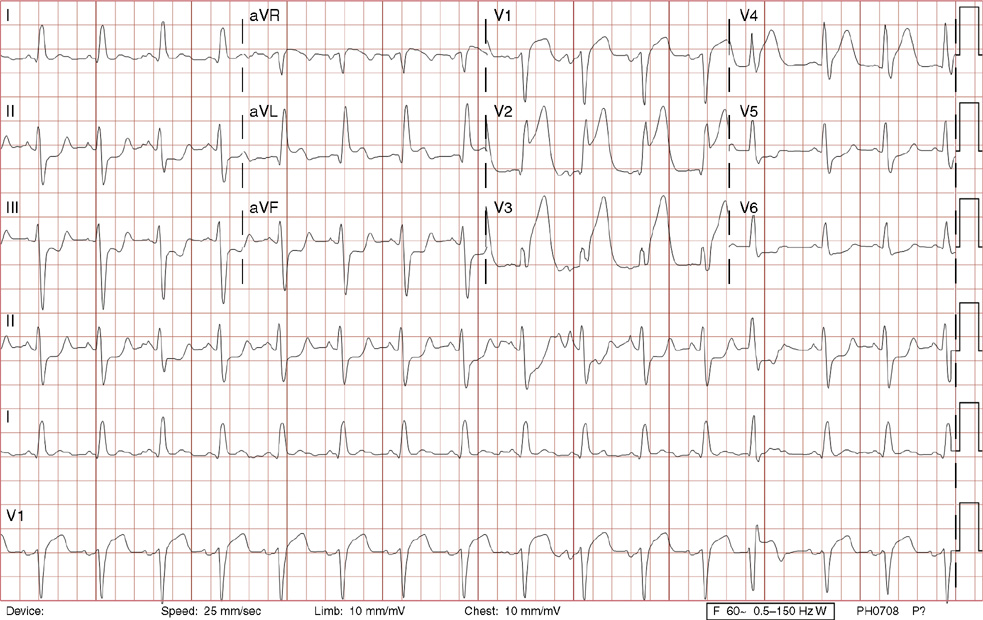
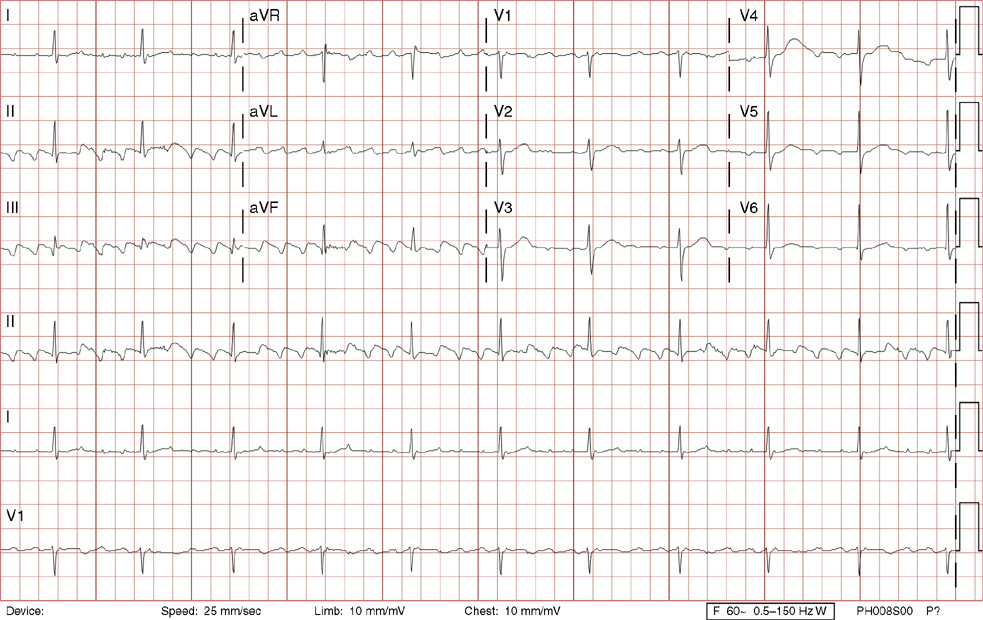
3. A 63-year-old man presents to your office with complaints of intermittent lightheadedness without syncope. He has type 2 diabetic, is hypertensive, and has gout but has no known cardiac disease. His ECG shows:
A. Sinus rhythm, first-degree AV block, intermittent blocked atrial premature complexes, right bundle branch block, and left anterior fascicular block
D. Sinus rhythm, third-degree AV block, ventricular escape rhythm
E. Accelerated idioventricular rhythm with AV dissociation
3. The answer is B: Sinus rhythm, Mobitz type I second-degree AV block, right bundle branch block, and left anterior fascicular block. This is bradycardia with an atrial rate just above 60 bpm, with lengthening of the PR interval before dropping QRS complexes. Note there are P waves seen at the end of the T waves of the 3rd, 5th and 7th beats (seen best in rhythm lead aVF), with no conduction after the 3rd and 5th beats, and even longer PR prolongation after the 7th beat, all consistent with Mobitz type I second-degree AV block. The QRS duration >120 ms (>3 small boxes), the rSR’ in lead V1, and the deep, wide S waves in lead V6 and lead I are all consistent with right bundle branch block. Left axis deviation in the absence of left ventricular hypertrophy, inferior infarct, or left bundle branch block is consistent with left anterior fascicular block. There is also left atrial enlargement defined by a P wave >120 ms (>3 small boxes) and/or two positive deflections separated by >40 ms (>1 small box) in lead II, and/or a Pwave with a negative deflection in lead V1 that is >40 ms (>1 small box) and deeper than 0.1 mV (>1 small box).
Mobitz type I second-degree AV block occurs within the AV node and is commonly asymptomatic, not requiring treatment. This patient was symptomatic with evidence of other conduction system disease (right bundle branch block and left anterior fascicular block) and therefore requires a pacemaker.
A. This is not first-degree AV block with blocked atrial premature contractions because none of the P waves are premature (they are regular), and the pattern of progressive PR lengthening before the dropped QRS complex is consistent with Mobitz type I second-degree AV block.
C. Mobitz type II second-degree AV block lacks progressive PR lengthening manifesting as random, unpredictable drops of QRS complexes. This degree of heart block is considered higher grade (below the AV node) and often will necessitate a pacemaker. Left posterior fascicular block, not present here, is defined as right axis deviation greater than 100 degrees in the absence of right ventricular hypertrophy, pulmonary embolism, lateral wall infarct, dextrocardia, or lead reversal.
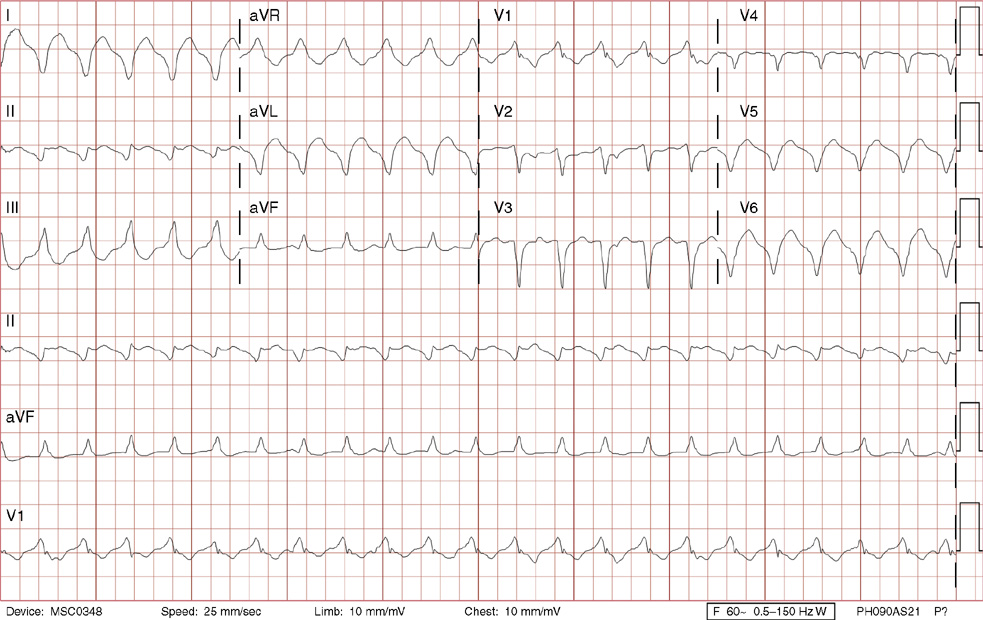
4. A 58-year-old man with a history of medically managed coronary artery disease, end-stage renal disease, hypertension, and diabetes presented with 2 weeks of intermittent shortness of breath with exertion. On the day of admission, he developed substernal chest discomfort described as, “Someone stepping on me.” In the emergency department, he was found to have a new left bundle branch block and went urgently to the catheterization lab where he was found to have a completely occluded proximal left anterior descending artery that was opened successfully with a bare metal stent. Thirty minutes after his intervention, the patient has this ECG done in the coronary care unit. What is the most reasonable next step?
B. Start intravenous (IV) amiodarone
C. Prepare the patient urgently for cardioversion
E. Have transcutaneous pacing pads placed on the patient, and the pacemaker on stand-by
4. The answer is A: Continue observation. This ECG reveals sinus rhythm, left atrial enlargement, first-degree AV block, left bundle branch block, prolonged QT, with the onset of an accelerated idioventricular rhythm (AIVR) starting with the fourth beat, and a ventricular premature complex (the last beat). Note there is AV dissociation, an indication of a ventricular source of arrhythmia, as the sinus P waves march through the rhythm strip at the bottom. By the 6th beat, the P wave is not responsible for the ventricular complex (the PR interval is too short), and by the 11th beat, you can see the P wave as a small negative deflection at the end of the QRS complex (see lead V1). The last recorded beat comes in early, but appears to be coming from the same focus as the AIVR. Note that it is followed immediately by what appears to be a retrograde P wave tucked in on the end of the QRS complex (best seen as a positive deflection in rhythm lead V1, and a negative deflection in rhythm leads II and aVF). There is then a compensatory pause with lack of regular sinus activity (blocked by the retrograde P wave). In AIVR, the ventricular rate is often between 60 and 110 bpm, frequently within 10 beats of the sinus rhythm with shift between the two occurring frequently as they compete for dominance. Fusion beats (4th and 5th beats) are QRS complexes with morphology between the ventricular beats and the normally conducted sinus beats as impulses from both fuse to form the complex, another marker indicating a ventricular source for the arrhythmia. AIVR is most often seen after an acutely occluded coronary artery is opened with thrombolytics or balloon angioplasty with or without stenting and is due to enhanced automaticity. It ordinarily requires no treatment and subsides on its own. Lastly AIVR can also be seen in digitalis toxicity, which can be treated with digoxin immune Fab (immunoglobulin fragments) acutely.
B, C, D, E. Ordinarily the patient is asymptomatic and requires no further treatment.
5. You are asked to see a 52-year-old woman with no prior medical history admitted to the neurosurgery service with a nontraumatic subarachnoid bleed. She was just placed on a ventilator, and an urgent craniotomy is planned. Based on the above ECG done 2 hours earlier, what would be the next best step before proceeding to surgery?
A. Echocardiogram to assess for right ventricular hypokinesis
C. Look at the chest x-ray to confirm a congenital heart abnormality
D. Stress test to assess for significant ischemia
5. The answer is B: Repeat ECG. This ECG demonstrates sinus tachycardia with apparent right axis deviation. However, make note that the predominantly positive precordial P waves appear to support a sinus mechanism from the high right atrium, but the negative P waves in I and flat P waves in II do not. This suggests limb lead reversal. With right arm–left arm lead reversal, as in this case, lead I is inverted, leads II and III are reversed, leads aVR and aVL are reversed, and lead aVF is unchanged. Right arm left–arm lead reversal is one of the more common reasons for right axis deviation, and assessing the P wave morphology as explained helps distinguish this. This abnormality needs to be distinguished from rare dextrocardia, which can also produce these limb lead findings. However, the precordial leads would show lack of normal R wave progression as the heart would be in the other side of the chest.
D. There is no role for a stress test in this patient who has an urgent noncardiac surgical indication.
E. There are no findings to suggest that β-blocker therapy is indicated.
6. A 24-year-old female heroin abuser presents with fever and malaise. She has been complaining of mild palpitations. She is normotensive, has a normal pulse oximetry on room air, but has a holosystolic murmur heard at the right lower sternal border. An ECG is performed. What would be the best next step?
D. Have transcutaneous pacing pads placed on the patient, and the pacemaker on standby
6. The answer is B: Echocardiogram. This patient’s ECG demonstrates sinus arrhythmia, which commonly occurs in the younger patient, and is due to normal, enhanced vagal tone. The P-P interval shortens during inspiration, due to reflex inhibition of vagal tone, and lengthens during expiration. Very rarely, sinus arrhythmia is symptomatic if the P-P interval is excessively long. Following blood cultures and antibiotic initiation, a transthoracic echocardiogram is the most reasonable next step given the IV drug abuse history, the fever, and the murmur suggestive of tricuspid valve regurgitation.
A. There is no reason to immediately repeat an ECG. Daily ECGs, however, are reasonable if there is concern or confirmation of aortic valve vegetations, as these could progress to aortic valve abscess and AV nodal block, often first manifest as first-degree AV block.
C, D, E. There is no immediate indication for atropine, transcutaneous pacing, or CT scan.
7. A 23-year-old man with no past medical history presents to the emergency department with syncope. He has a normal physical exam. Based on the ECG, the most appropriate next step would be:
A. Placement of a transvenous pacemaker
B. Referral for coronary angiography and angioplasty if indicated
C. Initiation of IV amiodarone
D. Referral for neurologic evaluation and electroencephalography (EEG)
E. Referral for electrophysiology study
7. The answer is E: Referral for electrophysiology study. This ECG shows sinus bradycardia, a delta wave consistent with preexcitation from a bypass tract (short PR interval, slurring of the initial QRS), and a pseudoinfarct pattern inferoposteriorly (Q waves and ST elevation in II/aVF). The presence of a preexcitation pattern with documented arrhythmias such as AVRT is known as Wolf-Parkinson-White syndrome. Given this ECG pattern and a history of syncope, there is high concern for more lethal arrhythmias that involve the bypass tract, such as atrial fibrillation that could conduct so quickly down the bypass tract to the ventricles as to cause VT, ventricular fibrillation, and sudden death. An electrophysiology study is indicated with possible radiofrequency ablation of the bypass tract.
A. There is no indication for transvenous pacemaker based on this ECG.
C. Amiodarone would be a good choice if there was evidence of rapid atrial fibrillation or AVRT, neither of which have been documented yet in this patient.
D. Neurologic evaluation with EEG is certainly a reasonable step in many patients with syncope. However, with this ECG pattern, cardiac syncope is more likely.
8. A 25-year-old man complains of 2 years of mildly progressive dyspnea on exertion. He presents for further evaluation, mostly at the insistence of his fiancée. His exam is notable for a prominent S2 heart sound and a systolic ejection murmur at the left upper sternal border with a subtle heave noted on palpation of the precordium. His ECG shows:
A. Sinus arrhythmia, right axis deviation, right atrial enlargement, right ventricular hypertrophy with strain pattern
D. Wandering atrial pacemaker, right bundle branch block
E. Sinus rhythm, left axis deviation, ischemic T wave abnormalities
8. The answer is A: Sinus arrhythmia, right axis deviation, right atrial enlargement, right ventricular hypertrophy with strain pattern. This pattern is consistent with right ventricular hypertrophy. There is right axis deviation defined as a QRS axis >100 degrees: note the S wave is deeper than the height of the R wave in lead I with predominantly positive QRS complexes in the inferior leads. Right atrial enlargement is defined as a tall P wave >0.25 mV (>2.5 small boxes) in lead II (seen here), and/or a tall P wave in lead V1 >0.15 mV (>1.5 small boxes). Right ventricular hypertrophy is seen with a prominent R wave that is taller than the small S wave in lead V1, relatively deep S waves in leads V5 and V6, a tall dominant R wave in lead aVR, with deep T wave inversions in leads V1 to V4 consistent with a strain pattern. On echocardiogram, this patient was found to have an enlarged right atrium, a hypertrophied and dilated right ventricle, and a pulmonary artery systolic pressure of 80 mm Hg. He was diagnosed with cor pulmonale secondary to idiopathic pulmonary arterial hypertension.
B, C, D, E. The rhythm is irregular due to sinus arrhythmia, not premature atrial contractions or wandering atrial pacemaker. The axis is not leftward given the predominantly negative deflection in lead I. Sometimes cor pulmonale can be associated with an incomplete or complete right bundle branch block. However, as in the ECG above, there is no true rSR’, and the QRS is narrow, excluding right bundle branch block. The notching of the downslope of the R wave in lead V1 is consistent with a delayed intrinsicoid (delayed time of the downslope after the R wave) which is also seen in right ventricular hypertrophy. Though the P wave in lead V1 appears to be deeper than 0.1 mV (>1 small box) it is not quite as wide as 40 ms (>1 small box), making this borderline left atrial enlargement. The precordial T wave abnormalities are more consistent with a right ventricular strain pattern from the right ventricular hypertrophy in this ECG rather than ischemia.
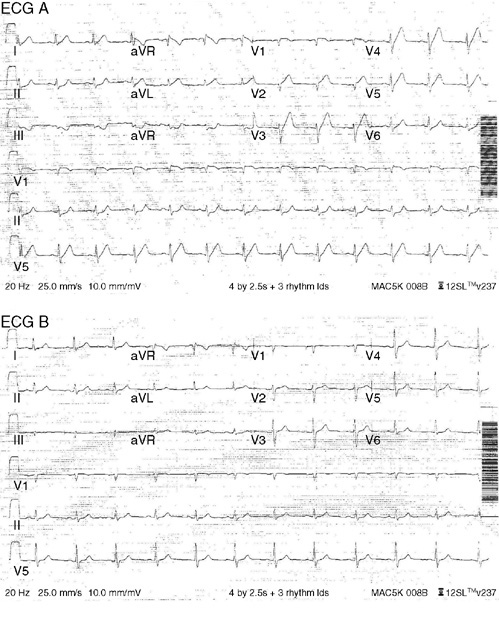
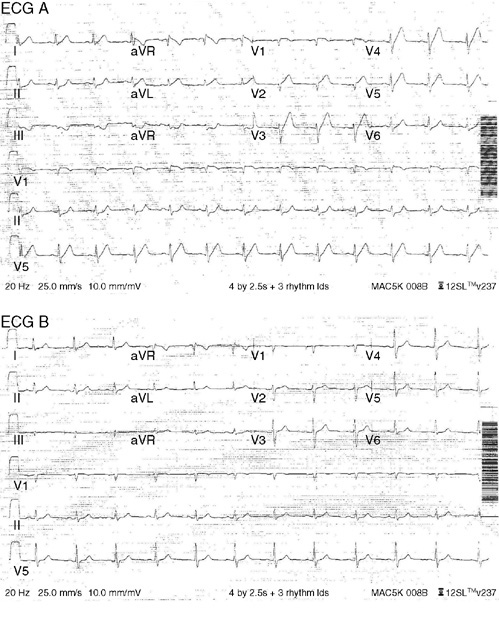
9. A 60-year-old man with hyperlipidemia taking over-the-counter fish oil supplements, and with a family history of a younger brother who had coronary artery bypass surgery, presented to an emergency department after experiencing jaw pain and upper chest tightness that started at the end of a routine 20-mile bike ride. ECG A was done at presentation (with chest pain), while ECG B was done 5 minutes later after sublingual nitroglycerin resolved the pain. Based on these ECGs, the most likely diagnosis is:
A. High grade left anterior descending artery stenosis
B. High grade right coronary artery stenosis
9. The answer is A: High grade left anterior descending artery stenosis. ECG A shows sinus rhythm with broad based prominent T waves in the precordial leads (called hyperacute T waves), inferior lead ST segment depression, and ST segment elevation in lead aVR, all consistent with diffuse subendocardial ischemia, likely from a high grade left anterior descending artery stenosis. Comparing ECG A to a prior ECG, or in this case, an ECG done 5 minutes later (ECG B) once chest pain resolved is important in identifying these dynamic ECG changes. ECG B shows resolution of the T wave and ST segment changes. The patient went on to the catheterization lab and a 70% mid-left anterior descending artery stenosis with associated thrombus was successfully stented. His precatheterization troponin was mildly elevated, and he did have mid- to distal-anteroseptal, anterior, and apical akinesis on echocardiogram due to myocardial stunning during ischemia. When ischemia is likely by clinical history and hyperacute, negative, or biphasic T waves are present in the precordial leads, this suggests high grade left anterior descending artery stenosis and should be treated aggressively.
C. The more broad based, rounded T waves (hyperacute T waves) here are not consistent with hyperkalemia, which usually would have sharp symmetrically peaked T waves, shortening of the QT interval, and sometimes ST segment elevation.
D, E. Hyperventilation and acute pulmonary embolus can cause ST segment and T wave changes, but not typically hyperacute T waves.
10. You are called to see your patient in the emergency department. He is a 54-year-old man with a history of abnormal calcium score on a prior cardiac CT, hypertension on treatment, and a family history of premature coronary artery disease in his younger brother. He presents with severe chest pain and upper back pain described as a “clamp” that began 30 minutes earlier. His blood pressure is 198/96 mm Hg in the right arm, and 170/76 mm Hg in the left arm. His exam is otherwise unremarkable except for diaphoresis and anxiety. An ECG is performed. Which would be the most appropriate next immediate step?
C. Start IV heparin now, followed by IV eptifibatide if his troponin is elevated
10. The answer is D: A chest x-ray. This ECG reveals sinus rhythm with ST elevation consistent with an acute inferior injury pattern and reciprocal lateral ST depression. In the vast majority of cases, there should be no delay in opening the occluded vessel with either IV thrombolytics (under 30 minutes from presentation) or coronary angioplasty and stent (under 90 minutes). Before proceeding emergently, however, it is always imperative to exclude any contraindications. In this patient with chest and back pain and a more than 20 mm Hg difference in arm blood pressures, ascending aortic dissection needs to be rapidly excluded. If a widened mediastinum is noted on chest x-ray, then emergent confirmatory imaging with either CT angiography of the aorta or transesophageal echocardiography could be done while cardiothoracic surgery is emergently consulted for a life-threatening aortic dissection. Alternatively, an aortogram could be performed at the beginning of a cardiac catheterization. If the mediastinum was not widened on chest x-ray, but the clinical suspicion remains high, further diagnostic studies should be rapidly performed before giving thrombolytics, anticoagulation, or IV antiplatelet agents. In this case, an aortic dissection was confirmed on CT angiogram, and the patient was transported to a hospital with a cardiothoracic surgeon. Ascending aortic dissection carries a mortality rate that increases approximately 2% per hour after presentation. Aortic dissections can sometimes present with an acute injury pattern such as this if the dissection flap occludes flow into the right coronary artery (which occurs more commonly than into the left).
A. There is no role for a V/Q scan to look for pulmonary embolus in this scenario.
B, C. IV thrombolytics are absolutely contraindicated in suspected aortic dissection, and IV heparin could worsen the dissection acutely.
E. IV nifedipine would not be a good choice as this could actually cause a reflex increase in cardiac contractility, possibly worsening the extent of an aortic dissection. A continuous IV β-blocker would be the first agent of choice to rapidly control the blood pressure while diagnostic tests are performed.
11. A 37-year-old male with a history of seizures since age 15 and migraines, presents to your office with complaints of palpitations that awoke him from sleep the night before and associated near-syncope. This occurred off and on over a few hours. He has had similar symptoms sporadically in the past several years, but this episode was more intense. He describes them as different from his seizures. His exam is normal. Based on the ECG above, the most appropriate recommendation is:
A. 24-hour outpatient continuous ECG monitoring
C. Follow-up with his neurologist
E. Admission to the hospital for emergent coronary angiography and angioplasty if indicated
11. The answer is B: Electrophysiology study. An electrophysiology study is indicated in this particular patient with near-syncope because the ECG shows sinus rhythm with ST elevation in leads V1 and V2 consistent with a Brugada pattern. Brugada syndrome with this ECG pattern is associated with ventricular arrhythmias and sudden arrhythmic death. Classically it is characterized by a prominent coved ST elevation greater than 0.2 mV (>2 small boxes) ending with a negative T wave in leads V1 and V2. Alternatively, and more subtly, this ST elevation more than 0.2 mV (>2 small boxes) can then sag in the middle of the ST segment > 0.1 mV or < 0.1 mV (> or <1 small box) above baseline, ending with a positive or biphasic T wave (termed saddle-back variations). The prevalence in the United States is not well established, but in Asia this syndrome is the most likely cause of sudden death in men younger than 50 years of age. It is at least eight times more prevalent in men than in women. Syncope and sudden death are common. The family history is important, though the syndrome can occur sporadically. It is due to a mutation in the SCN5A gene that encodes for a voltage-gated sodium channel. Treatment is an implantable cardioverter-defibrillator to prevent sudden death.
A, C. Given the presyncope with this concerning ECG pattern, electrophysiology study is warranted before 24-hour continuous ECG monitoring or neurology follow-up.
D, E. There is no indication for a stress test or coronary angiography, as the history and ST abnormalities are not typical of ischemia.
12. A 49-year-old patient with a history of sickle cell disease and cocaine abuse was admitted with intestinal ischemia and underwent urgent exploratory laparotomy and resection of the terminal ileum. Two days postoperatively, an ECG is performed. What would be the most appropriate next best step?
A. Urgent coronary angiography
E. Pulmonary embolus protocol CT
12. The answer is B: IV calcium gluconate. This ECG demonstrates sinus tachycardia, borderline intraventricular conduction delay, and peaked T waves consistent with hyperkalemia from lactic acidosis and renal failure. Calcium gluconate antagonizes the effects of potassium on myocardium, rapidly reducing the likelihood of arrhythmias, but it does not actually lower the potassium. Other measures such as insulin and dextrose infusions, sodium bicarbonate, and high-dose inhaled β-agonists will lower potassium by driving potassium from the extracellular space into cells. Sodium polystyrene sulfonate binds potassium in the intestinal tract but takes the longest to act. Lastly, in renal failure, hemodialysis is sometimes indicated if medical therapy fails. Hyperkalemia has a typical ECG progression. With potassium levels between 6to 6.5 mEq/L, there are peaked T waves with a very pointy peak as in this example. From 6.5 to 7 mEq/L, there is PR prolongation with flattening then loss of Pwaves. Greater than 8 mEq/L, there is widening of the QRS progressing to a sine-wave morphology followed by ventricular fibrillation and asytole.
A, C, D, E. There is no role for coronary angiography, as the J-point elevation seen in leads V1 through V3 is likely due to the hyperkalemia. Additionally, the ST segment is not convex up as would be more typical of an acute injury pattern. IV magnesium sulfate, potassium phosphate, or a pulmonary embolus protocol CT are not indicated.
13. A 47-year-old woman with hypertension treated with atenolol presents to your office complaining of fatigue and dyspnea with exertion that she notices on her morning hikes through the woods over the last week. She has no complaints at rest. On review of systems, she does report rashes in the past, but she could not recall if they were bull’s eye–shaped. She does report frequently removing ticks from her dog. On exam, her pulse rate is slow, but there are no rashes. Based on her ECG, what would be the best recommendation?
A. Decrease the atenolol dose and follow up in 1 week
B. Admission to the hospital for placement of a permanent pacemaker
C. Admission to the hospital for observation and possible placement of a transvenous pacemaker
D. Check Lyme titers with close outpatient follow-up
E. Admission to the hospital for urgent coronary angiogram with angioplasty if indicated
13. The answer is C: Admission to the hospital for observation and possible placement of a transvenous pacemaker. The ECG shows sinus rhythm with left atrial enlargement, third-degree AV block, and a fascicular escape rhythm in the 30s. Given the high-grade AV block with an escape rhythm well below the AV node, she is at high risk for syncope and sudden death. Therefore admission to the hospital for consideration of a transvenous pacemaker is appropriate.
E. Although coronary artery disease and ischemia can lead to heart block, there is nothing in her history or ECG that warrants urgent coronary angiography.
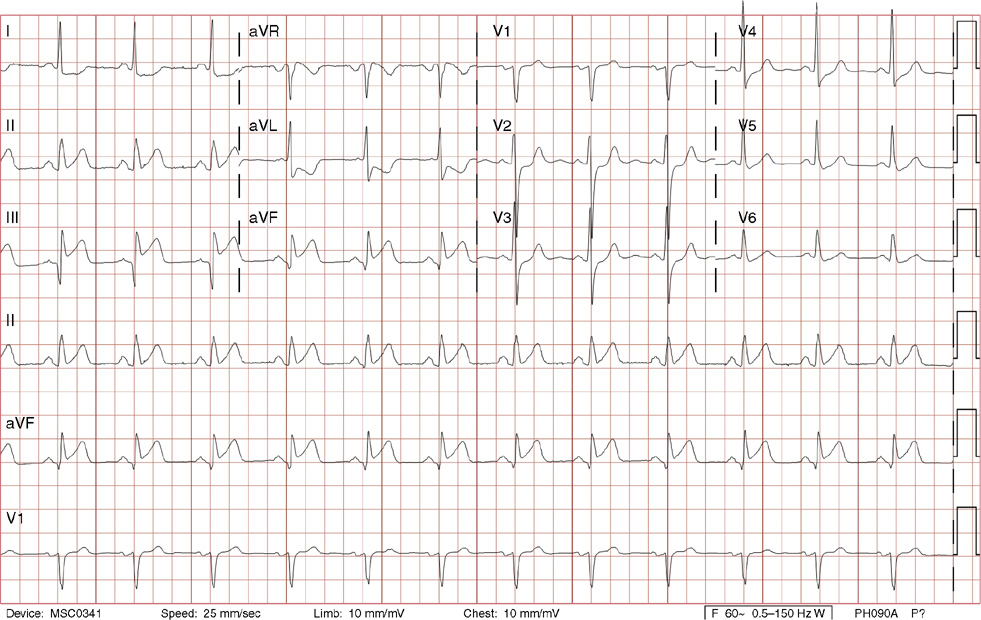
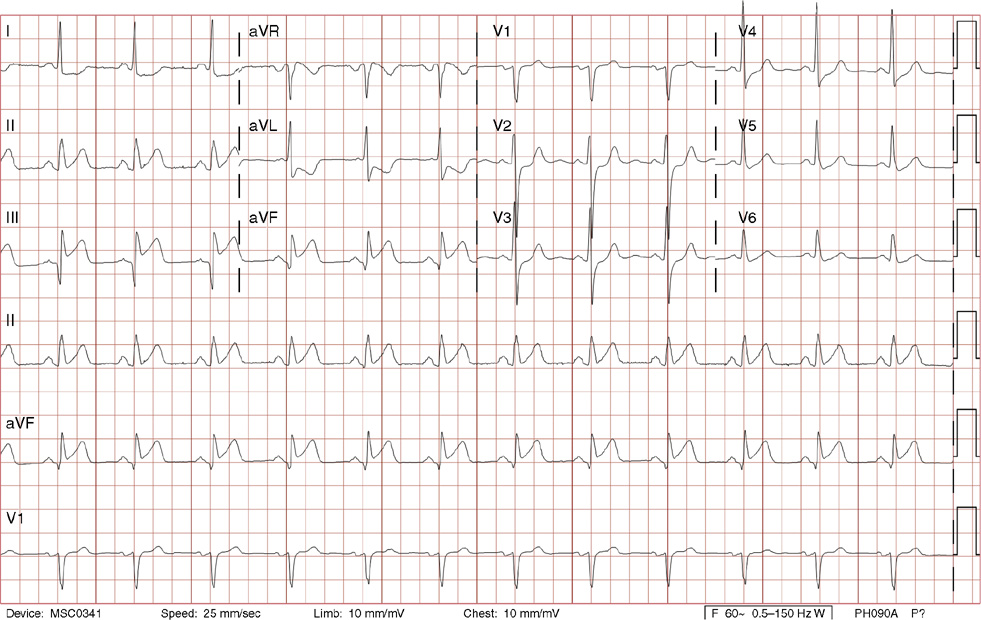
14. A 28-year-old man presents for a checkup after his 25-year-old brother died suddenly while diving in the Caribbean Islands. Your patient’s history is notable for one syncopal episode 4 years earlier, which was sudden and brief, without associated trauma. He did not seek medical care and thought it was due to heat exhaustion. He takes no medications and has no significant medical or social history. His exam is normal. His ECG is shown above. Which of the following can be associated with this ECG finding?
14. The answer is D: Erythromycin. The ECG shows normal sinus rhythm with a markedly prolonged QT interval. QT prolongation is associated with polymorphic ventricular tachycardia (torsades des pointes) and death. Given the patient’s age and family history of possible sudden cardiac death, congenital long QT syndrome is probable. There are other acquired causes of long QT, such as medications including erythromycin, clarithromycin, haloperidol, and methadone. For a complete list refer to www.torsades.org. In addition, other conditions may cause long QT such as ischemia, hypothyroidism, hypocalcemia, hypokalemia, and severe brain injury. The normal corrected QT interval is considered to be <460 ms for women and <440 ms for men. Visually, the QT interval should be less than 50% of the R-R interval. Congenital long QT syndrome may be associated with a family history of sudden death or syncope, especially before the age of 40. Long QT syndrome survivors of sudden cardiac death warrant an implantable cardioverter-defibrillator. Otherwise, β-blockers are the mainstay of prevention in most cases.
C. IV lidocaine and oral mexiletine are among a few antiarrhythmic medications that do not prolong the QT interval, unlike quinidine, procainamide, sotalol, and amiodarone.
15. A 56-year-old woman presents to her primary care physician complaining of 3weeks of new fatigue. She has rarely experienced a sense of dyspnea with exertion, but denies orthopnea, chest pain, presyncope, or syncope. She takes conjugated estrogens for hot flashes after an uncomplicated hysterectomy 10 years ago. Her exam is notable for a blood pressure of 150/88 mm Hg, but is otherwise unremarkable. An ECG is done in the office. The best next step would be:
A. Electrophysiology consultation for electrophysiology study
B. Lower extremity Doppler ultrasound
15. The answer is A: Electrophysiology consultation for electrophysiology study. The ECG shows sinus tachycardia just above 100 bpm with second-degree 2:1 AV block. The P waves that are not conducting can be seen at the end of every T wave (most evident here in leads I, V1, and V6). Because the block is 2:1, you cannot differentiate between Mobitz I or Mobitz II second-degree AV block. Mobitz I second-degree AV block, with progressive PR lengthening before dropping a QRS, is generally considered to be intranodal (within the AV node), typically stable, and rarely requires a pacemaker (only if very symptomatic). Mobitz II second-degree AV block has a stable PR interval but manifests as random, unpredictable dropping of the QRS complexes and is infranodal, usually unstable, and often requires a pacemaker. An electrophysiology study would be indicated, given her vague complaints, to differentiate the exact level of block and need for pacemaker.
C. Though she is hypertensive, atenolol is contraindicated in the setting of second-degree AV block.
E. Though heart block and dyspnea can be associated sometimes with ischemic heart disease, the next best test in this situation is an electrophysiology evaluation.
16. A 44-year-old man presents to the emergency department with 60 minutes of new chest fullness and diaphoresis followed by a brief loss of consciousness that began while he was carrying drywall on a job site. In the absence of any absolute contraindications, the best next step would be:
A. IV fibrinolytics under 30 minutes from arrival to the emergency department
B. Pulmonary embolus protocol CT
E. Antiinflammatory medications
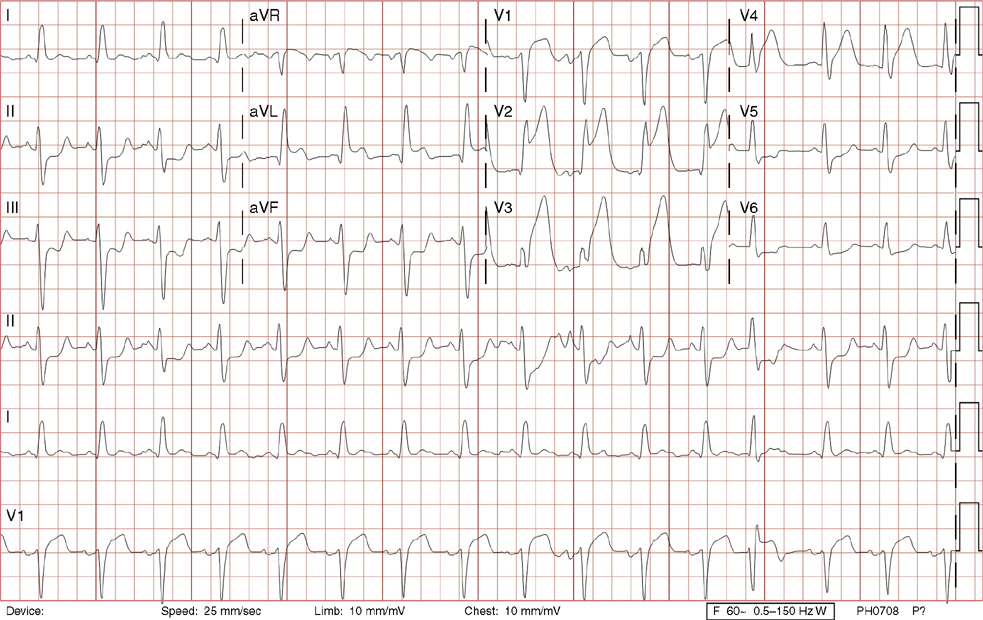
16. The answer is C: Coronary artery angiography with angioplasty with or without stenting under 90 minutes from arrival to the emergency department. This ECG shows an acute anteroseptal injury pattern with inferolateral reciprocal ST depression, consistent with an anteroseptal ST-elevation myocardial infarction. The basic rhythm is sinus rhythm with left atrial abnormality, left ventricular hypertrophy (R wave >0.11 mV [>11 small boxes] in lead aVL) with associated left axis deviation, early precordial R/S transition (as the QRS complex transitions from negative to positive earlier than lead V3), prolonged QT, a premature junctional beat (13th beat), and with slight aberrancy (incomplete RBBB pattern by the rsR’ pattern in V1, less than 120 ms [<3 small boxes]). Coronary artery angiography with angioplasty with or without stenting within 90minutes of arrival to the emergency department is the treatment of choice.
B, D, E. The symptoms and ST changes are not indicative of pulmonary embolus, chronic arrhythmias, or pericarditis, and therefore pulmonary embolus protocol CT, electrophysiology study, and antiinflammatory medications are not indicated. Syncope may be related to transient life-threatening VT, often associated with an acute infarction.
17. A 70-year-old woman with a history of coronary artery disease treated with prior coronary artery stents and known right bundle branch block presents to your office for a routine visit. She complains of new mild fatigue for 1 week. Her pulse rate is 130 bpm, blood pressure is 104/76 mm Hg. Her exam is otherwise notable for canon A-waves, variable S1, and a 2/6 holosystolic murmur at the apex. Her tachycardia prompts an ECG that shows the following:
A. Sinus tachycardia with right bundle branch block and left posterior fascicular block
C. Atrial flutter 2:1 conduction, with right bundle branch block and left posterior fascicular block
E. Sinus tachycardia with left bundle branch block
17. The answer is B: VT. The ECG demonstrates a wide complex regular tachycardia consistent with VT. There are two other major causes of wide complex tachycardias: any supraventricular tachycardia (including sinus tachycardia) with bundle branch block aberrancy and AVRT (also known as atrioventricular reciprocating tachycardia). Clinically the history of coronary artery disease makes any wide complex tachycardia more likely to be VT. Importantly, hemodynamic stability does not exclude VT. Three important telemetry strip or ECG findings that confirm VT over supraventricular tachycardia with aberrancy are AV dissociation, fusion beats, and capture beats. AV dissociation is noted here with supraventricular P waves seen between the 12th and 13th beats and the 14th and 15th beats are slightly more negative deflections than on rhythm lead V1. Note these P waves are seen in lead V2, as well. With calipers, you can march these P waves both forward and backward to find other confirmatory dissociated P waves at the same atrial rate (about 70 bpm) which is slower than the VT. AV dissociation leads to a variable sounding S1 as the mitral and tricuspid valves have variable degrees of opening depending on the timing of dissociated atrial contractions. Fusion beats are QRS complexes with morphology between supraventricular conduction and ventricular conduction as the two wave fronts meet to form one complex (not seen here). Capture beats occur when a dissociated P wave fortuitously finds the entire ventricle available for depolarization between VT beats, creating a normal native complex (not seen here). Other supporting findings to suggest VT are: an axis between –90 degrees and 180 degrees (northwest axis or far left axis); monophasic R wave in V1 (seen here); rSR’ in V1 with R’ wave taller than r; deeper S wave depth than R wave height; or QS wave in V6 (seen here). There are also other more advanced criteria called Brugada criteria and lead aVR criteria.
C. The absence of flutter waves excludes atrial flutter.
E. The positive deflection in V1 excludes left bundle branch block.
18. A 46-year-old woman with a history of systemic lupus erythematosus presents with acute onset chest pain beginning 5 hours ago. It began at rest, and she reports being unable to lie flat. Her younger brother had a myocardial infarction 1 year earlier. Her exam is notable for mild distress, blood pressure 112/78 mm Hg, and pulse oximetry 93% on room air. There is a faint malar rash, slightly decreased breath sounds at the bases of her lungs, with a regular heart rhythm without murmurs or gallops. There is no lower extremity edema. Based on her ECG, the best next step is:
A. Aspirin and urgent coronary angiography with possible angioplasty and stenting
B. IV heparin and pulmonary embolus protocol CT
C. Aspirin and IV thrombolytics
D. Aspirin and an echocardiogram
E. IV heparin and eptifibatide
18. The answer is D: Aspirin and an echocardiogram. High-dose aspirin or ibuprofen is the treatment of choice for pericarditis. In patients with a first episode of pericarditis, regardless of cause, the addition of colchicine appears to reduce the duration of symptoms and the recurrence rate. There is some suggestion that steroids are associated with a higher recurrence rate, though they may need to be given to treat an underlying autoimmune disease when present, or when aspirin or nonsteroidal antiinflammatory medications are contraindicated or ineffective. An echocardiogram is reasonable to assess for a pericardial effusion in this patient with lupus. The ECG shows diffuse ST segment elevation without reciprocal changes. ECG changes in acute pericarditis occur in four stages, but some may be missed. Stage one consists of concave upward ST segment elevation and PR segment depression in almost all leads except aVR. Stage two is when the ST segment returns to baseline with the onset of T wave flattening. Stage three consists of T wave inversions. Stage four is a return to the normal ECG. Classically the patient has worsening of symptoms when supine and relative relief when leaning forward, because the heart hangs from its attachment with less approximation of the heart with the pericardial sac walls in this position. A pericardial friction rub is often heard as a rasping or scratching sound, but may be absent or intermittent.
B. Though the patient has chest pain with a relatively low pulse oximetry, the ECG points toward pericarditis as a specific cause, therefore there is no role for pulmonary embolus protocol CT at this point.
19. A 51-year-old man with non-insulin–dependent diabetes, hypertension, hyperlipidemia, and chronic obstructive pulmonary disease (COPD) presents for a physical in order to apply for a life insurance policy. His medications include metformin, atenolol, hydrochlorothiazide, and tiotropium inhaler. An ECG is performed. The best next step is:
19. The answer is E: Warfarin. The ECG shows atrial flutter with 4:1 conduction. Warfarin is indicated given his history of diabetes and hypertension. Atrial flutter is a reentrant atrial arrhythmia which typically has an atrial rate of between 250 and 350 bpm. If this patient was not already taking atenolol, the atrial flutter may have conducted 2:1, resulting in a heart rate of about 150 bpm, which may make seeing the flutter waves more difficult. The most common form of atrial flutter has dominant negative flutter deflections in the inferior leads and positive flutter deflections in lead V1 due to counterclockwise rotation within the right atrium, passing through the cavotricuspid isthmus. Generally atrial flutter is acutely treated like atrial fibrillation. If unstable, cardioversion is indicated. If tachycardic, IV or oral rate controlling agents can be offered such as non-dihydropyridine calcium channel blockers, β-blockers, and less commonly digitalis. Anticoagulation decisions are similar to those for atrial fibrillation, though there are less data. Lastly, catheter based ablative therapies can be offered, particularly when the atrial flutter is counterclockwise involving the cavotricuspid isthmus, such as this.
C. Amiodarone or dronedarone are antiarrhythmic agents which should not be started without anticoagulation in this patient.
20. A 77-year-old woman with paroxysmal atrial fibrillation, diabetes, and breast cancer recently had her diltiazem increased and digoxin added 3 weeks earlier for rapid ventricular rates. Over the last several weeks, she has had progressive fatigue, anorexia, and several near-syncopal episodes. An ECG is performed in the office. Which of the following statements is most true regarding her diagnosis?
A. There is complete heart block.
B. Coronary angiography should be urgently performed.
C. Ventricular arrhythmias are uncommon.
20. The answer is D: Vision changes are common. This ECG reveals sinus bradycardia with a rate below 40 bpm, with ST segment changes consistent with digitalis effect. The constellation of fatigue, anorexia, and presyncope combined with this ECG revealing significant bradycardia raises the concern for digitalis toxicity. Vision changes are relatively common, including color vision complaints, scotomas and, rarely, blindness. With chronic toxicity, serum digitalis levels do not correlate with toxicity as asymptomatic patients can have elevated levels, and toxic patients could have therapeutic levels. Medications such as diltiazem, verapamil, amiodarone, and quinidine can increase digitalis levels, as can renal failure. Arrhythmias are common, including bradyarrhythmias (including complete heart block) due to excessive parasympathetic tone, junctional arrhythmias, ventricular ectopy, and VTs, including biventricular tachycardia, and classically, atrial fibrillation or flutter with AV block. ST segment changes from digitalis effect are a normal J point (the transition point between the QRS complex and the ST segment) followed immediately by a sagging or scooped ST segment. ECG digitalis effect alone does not indicate toxicity.
B. The ST segment changes are not typical of ischemia, but rather digitalis effect.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree