Increasing emphasis on tailoring of cancer treatment strategies to individual patients (i.e., personalized medicine) has enabled development of multiple therapeutic options in the management of malignant diseases of the abdomen and pelvis. In particular, in patients with malignant hepatic and renal tumors, organ-directed treatment such as percutaneous ablation, intra-arterial embolic therapies, and targeted radiation therapy have shown considerable promise in improving patient outcome. Although surgical resection is considered the gold standard treatment option for management of these tumors, percutaneous ablative therapies offer a valuable therapeutic option in renal cell cancers and hepatic malignancies. Intra-arterial therapies are effective treatment options in patients with locally advanced liver malignancy, and image-guided radiation treatments are suitable alternatives for unresectable hepatic malignancies or those that are difficult to ablate.
Imaging has a crucial role in the successful delivery of therapy in patients selected to undergo image-guided loco-regional therapies. The role of imaging before treatment is to determine tumor burden and staging; depict the anatomic relationships of tumor, particularly its proximity to critical structures; select the appropriate treatment option; and plan the treatment approach. After loco-regional therapy, imaging is not only required to monitor therapeutic success and detect residual and recurrent disease but is also essential to detect procedure-related complications. Continuing technologic advances in computed tomography (CT) and magnetic resonance imaging (MRI) not only permit reliable morphologic evaluation of hepatic and renal tumors but also enable functional assessment. Functional imaging techniques such as perfusion CT/MRI, diffusion weighted imaging (DWI), 18-fluorodeoxyglucose (FDG) with positron emission tomography (PET), and/or PET/CT enable evaluation of various physiologic properties of tumors, such as vascularity and metabolism, which are increasingly being used before and after loco-regional therapies. This chapter will briefly describe the role of imaging in preprocedural evaluation and postprocedural assessment of abdominal malignancies undergoing image-guided targeted therapies, with particular focus on hepatic and renal tumors.
Image-Guided Treatment Options
The past few decades have seen a substantial rise in the performance of image-guided loco-regional therapies for various tumors in the abdomen and pelvis. Prominent image-guided treatments include percutaneous image-guided ablation and intra-arterial therapies. Percutaneous image-guided ablative techniques include radiofrequency ablation (RFA), percutaneous ethanol injection, microwave ablation (MWA), cryoablation, laser ablation, and irreversible electroporation (IRE). Whereas percutaneous ablations are most commonly used for treatment of renal cell cancer and malignant liver lesions, intra-arterial and image-guided radiation therapy are primarily offered in the management of hepatic malignancies.
Percutaneous ablation is based on the principle of local tumor destruction through needle-guided administration of chemicals, heat, or electric current into the tumor while avoiding damage to the surrounding normal tissue. Although percutaneous ethanol injection achieves tumor destruction through slow image-guided instillation of absolute or 95% alcohol into the tumor resulting in coagulative necrosis, RFA causes tumor death by generating heat through transmission of high-frequency alternating current (AC). MWA works on the principle of tissue destruction through electromagnetic waves, with frequencies 90 kilohertz or greater inducing rapid molecular motion of cellular particles with heat generation and coagulative necrosis. IRE is a nonthermal ablation technique in which tissue destruction is achieved by permanent cell membrane permeability increase induced by powerful high-voltage electric pulses (3 kilovolts). Increased permeability permits transmembrane movement of water-soluble substances and ions, disrupting cellular homeostasis and causing cell death. Most ablation procedures are percutaneous, although laparoscopic and intraoperative approaches are also used. Among the various ablative techniques, the collective experience with RFA is the largest and RFA has come to be considered a standard treatment option for local tumor control.
In the treatment of hepatic malignancies, intra-arterial therapies such as transarterial chemoembolization (TACE) and selective internal radiation therapy (SIRT) are most frequently performed. These involve transarterial administration of different particles into vessels supplying the tumor to accomplish tissue destruction. Transarterial embolization techniques are possible as a result of the dual hepatic vascular supply (portal vein, 75%; hepatic artery, 25%), which enables selective intra-arterial instillation of embolic agents and chemotherapeutic drugs into the tumor because hepatic tumor blood supply is preferentially arterial. The intra-arterially administered substances cause obstruction of tumor feeding vessels and result in cell death while the normal liver parenchyma, which is supplied by the portal vein, is not damaged. SIRT or radioembolization involves intra-arterial administration of micron-sized particles (20 to 60 microns) containing a radioisotope (yttrium-90 [ 90 Y]), which delivers focused beta radiation to cause tumor destruction while minimizing radiation damage to the surrounding normal liver. In contrast to TACE, SIRT requires preservation of adequate perfusion to the tumor to enhance free radical–dependent cell death from radiotherapy. In hepatocellular carcinoma (HCC), intra-arterial therapies are considered the first-line palliative therapy for inoperable cases with large or multifocal HCC without major vascular invasion or extrahepatic disease but with preserved liver function and performance status. In hepatic metastases, intra-arterial therapies have a palliative role particularly in management of hepatic metastases from neuroendocrine tumor, breast cancer, and colon cancer.
Image-guided radiation therapy is increasingly used for management of hepatic malignancies because novel three-dimensional (3D) conformal radiation therapy allows targeted delivery of a high radiation dose to limited volumes of liver while limiting irradiation of residual functioning liver parenchyma. These techniques use percutaneously placed radiopaque fiducials around the tumor to maintain high precision in radiation delivery leading to DNA damage and causing cell death.
Imaging Protocol
Dynamic contrast multidetector CT (MDCT) and gadolinium-enhanced MRI are considered the standard of care for preprocedural assessment of abdominal tumors before loco-regional therapies, as well as for monitoring response to treatment. Although CT and MRI are equally accurate in pretreatment evaluation, gadolinium-enhanced MRI is preferred for evaluation of hepatic malignancies because of higher specificity and superior soft tissue and contrast resolution. For hepatic tumors, dynamic contrast-enhanced imaging with arterial phase, portal venous phase, and delayed phase is typically performed. In hepatic malignancies, particularly metastases from an extrahepatic primary, such as colorectal cancer, PET/CT is increasingly used for pretreatment assessment to identify extrahepatic sites of disease and for accurate tumor staging. For metastatic disease, PET/CT is also preferred over CT for evaluation of ablation zone and after SIRT therapy. Morphologic imaging studies such as CT and MRI are often insensitive compared to PET/CT for monitoring response after SIRT therapy because of the presence of necrosis, edema, hemorrhage, and cystic change.
For renal tumors, the typical protocol includes an unenhanced, nephrographic phase and delayed phase acquisition. The delayed phase is typically acquired to define the relationship of the tumor to the renal collecting system and detect urinary complications. Subtraction imaging adds value as a complementary technique for precise determination of enhancement in the treatment zone. Coronal and sagittal reformation images are also obtained. Irrespective of the technology used for surveillance, it is essential to maintain consistency in the imaging protocol performed at baseline before therapy and at different time points after therapy. The initial timing of imaging assessment after various loco-regional therapies is generally 4 to 8 weeks after treatment. Following initial assessment of treatment response, subsequent imaging follow-up is performed to detect local tumor progression evidenced by recurrence and development of new local and distant sites of disease. Our current practice includes posttreatment imaging follow-up at 1, 3, 6, 9, and 12 months, followed by imaging at longer intervals if no residual or recurrent disease is revealed.
Imaging Protocol
Dynamic contrast multidetector CT (MDCT) and gadolinium-enhanced MRI are considered the standard of care for preprocedural assessment of abdominal tumors before loco-regional therapies, as well as for monitoring response to treatment. Although CT and MRI are equally accurate in pretreatment evaluation, gadolinium-enhanced MRI is preferred for evaluation of hepatic malignancies because of higher specificity and superior soft tissue and contrast resolution. For hepatic tumors, dynamic contrast-enhanced imaging with arterial phase, portal venous phase, and delayed phase is typically performed. In hepatic malignancies, particularly metastases from an extrahepatic primary, such as colorectal cancer, PET/CT is increasingly used for pretreatment assessment to identify extrahepatic sites of disease and for accurate tumor staging. For metastatic disease, PET/CT is also preferred over CT for evaluation of ablation zone and after SIRT therapy. Morphologic imaging studies such as CT and MRI are often insensitive compared to PET/CT for monitoring response after SIRT therapy because of the presence of necrosis, edema, hemorrhage, and cystic change.
For renal tumors, the typical protocol includes an unenhanced, nephrographic phase and delayed phase acquisition. The delayed phase is typically acquired to define the relationship of the tumor to the renal collecting system and detect urinary complications. Subtraction imaging adds value as a complementary technique for precise determination of enhancement in the treatment zone. Coronal and sagittal reformation images are also obtained. Irrespective of the technology used for surveillance, it is essential to maintain consistency in the imaging protocol performed at baseline before therapy and at different time points after therapy. The initial timing of imaging assessment after various loco-regional therapies is generally 4 to 8 weeks after treatment. Following initial assessment of treatment response, subsequent imaging follow-up is performed to detect local tumor progression evidenced by recurrence and development of new local and distant sites of disease. Our current practice includes posttreatment imaging follow-up at 1, 3, 6, 9, and 12 months, followed by imaging at longer intervals if no residual or recurrent disease is revealed.
Imaging: Pretreatment Evaluation
Imaging is integral to the multidisciplinary care of patients diagnosed with hepatic or renal malignancy because patient selection is often carried out depending on the oncologic assessment, as well as the technical feasibility of various treatment options. Detailed radiologic evaluation before therapy is crucial to selecting the most appropriate local or systemic approach—that is, surgical resection versus systemic chemotherapy versus loco-regional therapy. Preprocedure imaging with CT or MRI allows tumor localization, depicts tumor margins and relationship to adjacent structures, and determines a safe path for percutaneous intervention, protecting and minimizing damage to adjacent normal and critical structures. * Pretherapeutic evaluation also permits assessment of tumor vascularity and metabolic activity to ensure precise dose and delivery of targeted therapies. The next few paragraphs will discuss several points requiring careful attention during assessment before loco-regional therapies.
* References .
Tumor Staging
A key function of imaging is the accurate determination of tumor stage, including evaluation of tumor size, number, location, presence of major vascular involvement, nodal disease, and distant metastases to select the most optimal targeted therapeutic option. In patients with hepatic tumors, the size and number of lesions dictate the type of loco-regional therapy with solitary tumors or fewer than 3 tumors being treated by percutaneous ablation or radiation therapy and multiple hepatic lesions are managed with intra-arterial therapies. For percutaneous ablation, tumor size has considerable influence on the treatment plan, including determination of types and number of electrode probes (single vs. clustered probes). Tumor size is also a strong predictor of treatment success and survival for both hepatic and renal tumors.
In patients with HCC, tumors 5 cm or less have a higher likelihood of achieving a favorable outcome with percutaneous ablation and in tumors less than 3 cm the outcomes after RFA reportedly match those with surgical resection ( Figure 86-1 ). Large HCCs measuring more than 5 cm are generally not considered ideal candidates for percutaneous ablation, and surgical resection remains a favored option. A combination of TACE and percutaneous ablation may benefit patients with tumors greater than 5 cm. Patients with multinodular or multifocal HCC and relatively preserved liver function without major vascular invasion or extrahepatic disease are often treated with intra-arterial therapies. In patients with oligometastatic liver disease (solitary tumor or <5 tumors measuring ≤3 cm), ablative therapies, and image-guided radiation therapy are increasingly offered as an option to patients who refuse surgery or when surgery is contraindicated. In these patients, RFA has shown some success in controlling tumor burden, with 5-year survival rates of 24% to 44% for tumors less than 5 cm and favorable outcome in metastases less than 3 cm (5-year survival rate of 55% to 56%). † Tumor recurrence rates after RFA in metastatic colorectal cancer vary from 6% to 40% and are relative to size, number, and location of lesions with worsening outcome for tumors greater than 5 cm. Higher rates of complete ablation can be achieved with MWA because of the larger zones of ablation achieved. In patients in whom ablation is not feasible or an option, intra-arterial approaches such as TACE (with irinotecan) and SIRT therapy are other promising but less established approaches. In hepatocellular carcinoma, intra-arterial therapies are offered as the first-line palliative therapy for nonsurgical patients with large or multifocal HCC without major vascular invasion or extrahepatic disease but with preserved liver function and performance status (see Figure 86-1 ).
† References .
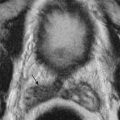
In RCC, tumor size dictates treatment success, with optimal tumor size for percutaneous ablation being 4 cm or less or T1a tumors according to American Joint Committee on Cancer staging ( Figure 86-2 ). In tumors less than 3 cm, complete tumor necrosis can be achieved, resulting in successful treatment in 100% of cases. Increasing tumor size limits treatment effectiveness with reduced likelihood of recurrence-free survival, thereby necessitating an increased number of ablation sessions to achieve an equivalent degree of necrosis. Due to the ability to visualize the treatment zone, cryoablation decreases the number of sessions needed for successful treatment of the tumor. The tumor size also dictates the type of electrode, number of overlapping ablations, and number of treatment sessions. Whereas tumors measuring 1.5 cm can be treated with a single ablation with a cluster electrode, increasing tumor size necessitates use of more electrodes, overlapping ablations, and multiple sessions.
Tumor Location
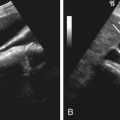
Tumor location has a significant impact on loco-regional treatment strategy because it determines the type of therapy and the percutaneous approach to the tumor. Precise delineation of tumor location and its relationship to adjacent structures is mandatory before percutaneous ablation because it not only determines a safe trajectory to the tumor but also influences planning of the procedure, including patient position, type of electrode, and need for adjunctive procedures such as hydrodissection. The anatomic relationship of the tumor to surrounding structures such as the stomach, duodenum, and colon should be ascertained before ablation because it not only limits the ability to perform complete ablation, thereby affecting treatment efficacy, but also potentially increases the complication rate, such gastrointestinal perforation. Adjunctive procedures such as hydrodissection (or intraperitoneal instillation of dextrose fluid before thermal ablation) is often performed in these circumstances to move the bowel loops away from the renal or hepatic tumor and thereby limit injury to these organs from ablation ( Figure 86-3 ). Additionally, other ablative procedures such as alcohol injection or IRE could be performed to limit adjacent organ injury.

Careful attention to the relationship of hepatic and renal tumors to structures with the liver and renal hilum is essential to avoid inadvertent injury to the biliary tract and renal collecting system or ureter, respectively. Percutaneous ablation of tumors situated within less than 1 cm from large biliary ducts has been reported to cause biliary stenosis. Similarly, ablation of central renal tumors could lead to collecting system injury, including infundibular or ureteral strictures and urinoma formation. Retrograde pyeloperfusion is an effective adjunctive procedure used in percutaneous ablation of central renal tumors to minimize collecting system and ureteral injury (see Figure 86-3 ). Image-guided ablation of tumors close to the hepatic and renal vessels is feasible, and thermal injury to these vessels is limited because of blood flow. However, this potentially can limit the efficacy of RFA as a result of possible “heat sink effect.” This effect can lead to incomplete treatment of tumor tissue, and local recurrence rates of up to 48% have been reported for tumors close to large vessels. In exophytic renal tumors, the “oven effect” of surrounding perinephric fat has been reported to “contain” heat within the tumor, leading to enhanced tumor ablation. In patients scheduled for image-guided radiation treatment, close proximity of the hepatic tumors to structures such as breast, stomach, and small or large bowel can increase nontarget organ radiation injury and surgical placement of AlloDerm spacers (LifeCell, Bridgewater, NJ) to move the structures away from radiation field has been documented.
Local Tumor Invasion and Metastatic Disease
Loco-regional therapies generally are not indicated for locally advanced tumors with major vascular invasion because it adversely affects outcome and overall survival ( Figure 86-4 ). Multiplanar reformations and 3D reconstructed images are very useful to accurate depiction of vascular involvement (see Figure 86-4 ). Tumors with infiltrative margins are less likely to be successfully treated with percutaneous ablation compared with well-encapsulated tumors. In hepatic malignancies, the presence of biliary involvement such as biliary invasion or duct dilatation should be identified before considering ablative or intra-arterial therapies, because these procedures increase the risk for biliary necrosis and infective complications. Presence of distant metastases adversely affects outcome and is a relative contraindication for loco-regional therapies because these treatment options are generally considered for local tumor control.

Vascular Anatomy
Intra-arterial therapies such as TACE and SIRT necessitate evaluation of the arterial vascular anatomy before embolization procedures. Preprocedural evaluation of normal and variant vascular anatomy should be performed along with identification of preexisting vascular diseases such as arterial atherosclerosis for treatment planning. During embolization procedures, it is crucial to identify collateral vascular supply to hepatic tumors to ensure complete tumor embolization. Before the SIRT procedure, it is mandatory to perform a pre-SIRT baseline diagnostic angiogram to map the mesenteric vascular anatomy to identify normal and variant vascular anatomy and hepatofugal flow. This step is very important because prophylactic embolization of vessels such as gastroduodenal and right gastric arteries is required to prevent nontarget radioactive embolization of stomach and small bowel, which can result in intractable radiation ulcers. In addition, single-photon emission CT (SPECT) imaging with macroaggregated albumin particles injected into the hepatic artery is also performed to detect arteriovenous shunting into the gastrointestinal or pulmonary vasculature. Recognition of any collateral vasculature with liver-directed flow at the same time as the presence of any relevant arteriovenous fistulae should prompt embolization before SIRT therapy to improve treatment effectiveness. Severe lung shunting that cannot be treated by embolization is a contraindication to SIRT.
Tumor Metabolism
18-FDG PET is a noninvasive metabolic imaging technique used in the pretreatment and postprocedural evaluation of patients undergoing loco-regional therapies. PET/CT scanners, which combine the metabolic information of 18-FDG-PET with the precise anatomic information provided by CT, allow precise lesion localization. Use of PET/CT for pretreatment evaluation is beneficial in tumors that demonstrate FDG uptake because this permits monitoring of treatment response ( Figure 86-5 ). PET/CT is a useful tool for detection of unsuspected extrahepatic metastatic disease, which often precludes loco-regional treatment options. Although the utility of PET/CT in HCC is limited as a result of variable FDG uptake, PET/CT is very useful in patients with colorectal metastases treated with MWA, RFA, or TACE. PET/CT has been reported to be better than CT alone for detection of extrahepatic disease and improves staging in colorectal cancer, thyroid cancer, melanoma, and carcinoid tumors. PET/CT, however, does not presently have a role in the pretreatment evaluation and staging of renal cell cancer.

Estimation of Functional Reserve
The main advantages of loco-regional therapies are the benefits of limited damage to the surrounding normal hepatic and renal parenchyma and tumor destruction. A favorable long-term outcome relies not only on successful tumor destruction but also on adequate residual functional parenchyma. Therefore, assessment of organ function is essential before undertaking loco-regional therapies. Whereas laboratory tests such as serum urea, creatinine, transaminases, and so forth can provide a reliable estimation of organ function, the value of imaging in determining functional reserve is often underestimated. It is important to pay particular attention to renal or liver dysfunction during pretreatment assessment and appropriately comment on these facts in the radiology reports. Reliable estimation of the functional reserve helps in triaging patients into surgery versus local ablative therapies. Patients with inadequate functional reserve might be poor surgical candidates because of the propensity to develop organ failure. This is important in patients with severe fibrosis or cirrhosis or with solitary kidney, in which ablative treatment is preferred because of suboptimal functional reserve. Appropriate liver reserve is a prerequisite for SIRT procedures because hepatic parenchymal damage can result from radiation treatment. In patients with liver cirrhosis, imaging can help in reliable determination of hepatic functional reserve by recognition of features such as ascites or hydrothorax, markers of portal hypertension such as splenomegaly, and esophageal or gastric varices. Identification of these imaging features usually necessitates additional procedures before management of cancer, such as paracentesis, endoscopic variceal ligation, and so forth. Another factor in determination of background parenchymal changes is the impact of these changes on success of percutaneous ablative therapies. For example, thermal ablation of hepatic tumors is likely to be more efficacious in the setting of background cirrhosis because of the insulating effect of the fibrotic liver (oven effect) leading to elevated intratumoral temperatures and longer cytotoxic effect.
Imaging: Posttreatment Evaluation
Imaging has a cardinal role in the postprocedural management of patient treatment with loco-regional therapies for malignancies of the abdomen and pelvis. The four principal goals of imaging follow-up after treatment are as follows :
- 1.
Define the expected normal changes at the treatment site
- 2.
Identify abnormal changes such as residual disease or tumor recurrence
- 3.
Permit recognition of treatment-related complications
- 4.
Detect new areas of disease distant from the ablation zone, including extrahepatic disease
Early identification of residual recurrent disease and complications helps guide appropriate intervention and additional treatment sessions. Typically, the first postprocedure imaging evaluation is performed 4 weeks after completion of treatment. At this time, the patient is typically seen by an interventional radiology physician who reviews the imaging with the patient. Subsequent follow-up imaging is performed more closely during the first year because of a higher likelihood of recurrence in the initial year after treatment.
Imaging Appearances
Percutaneous Ablation
The imaging appearances after ablation of renal and hepatic tumors are similar. The expected changes vary in appearance based on the time elapsed after the procedure and evolve over time. Irrespective of the ablative technology, it is important to remember that 5 to 10 mm of normal parenchymal tissue is included in the ablation zone to ensure a tumor-free margin and effectively eliminate the microscopic tumor invasion usually existent around the tumor periphery while preserving normal organ function. Therefore, the size of the final ablation zone is usually larger than the tumor dimensions before treatment. The ablation zone in the immediate posttreatment scan classically appears as a nonenhancing region with areas of hyperdensity on CT resulting from the presence of proteinaceous material or hemorrhage ( Figures 86-6 and 86-7 ). Also very commonly seen are small air bubbles in the ablation zone secondary to tissue necrosis, including occasional small foci of gas in the portal vein. In postcontrast images, completely treated hepatic tumor demonstrates a total lack of enhancement on arterial, portal venous, and delayed phase images. A thin rim of peripheral enhancement is often seen around the ablation zone that manifests a physiologic inflammatory response to thermal injury caused by granulation tissue surrounding the zone of intratumor coagulation necrosis. This benign rim enhancement is transient, with a uniform appearance and smooth inner margins, and should be differentiated from the irregular nodular enhancement of residual tumor seen at the periphery of the ablation zone. On MRI, the hepatic ablation zone has a heterogeneous appearance on T1-weighted images with description of three zones: a central zone of hypointensity surrounded by a broad hyperintense zone capped by a hypointense band. The hypointense band represents areas of sinusoidal congestion in the acute phase and fibrotic change in the subacute phase. On T2-weighted images, the treated zone appears predominantly hypointense because of dehydration and coagulative necrosis. However, hyperintense foci secondary to hemorrhage can be seen. After gadolinium administration, the ablation zone demonstrates absence of enhancement. It is not uncommon to see perfusional changes in the liver after ablation, which include wedge-shaped peripheral areas of arterial enhancement adjacent to the ablation zone resulting from arteriovenous shunting from ablation injury. When in doubt, the ablation zones can be observed on close-interval follow-up examinations. Whereas recurrent tumors show interval growth, perfusional variants disappear or become smaller on follow-up imaging.