Intracranial aneurysms are common in the adult population and carry a risk of rupture leading to catastrophic subarachnoid hemorrhage. Treatment of aneurysms has evolved significantly, with the introduction of new techniques and devices for minimally invasive and endovascular approaches. Follow-up imaging after aneurysm treatment is standard of care to monitor for recurrence or other complications, and the preferred imaging modality and schedule for follow-up are areas of active research. The modality and follow-up schedule should be tailored to treatment technique, aneurysm characteristics, and patient factors.
Key points
- •
Management of intracranial aneurysms is complex, with factors including recent rupture, patient factors, aneurysm size, shape, and location affecting the decision whether and how to treat.
- •
Progress in endovascular aneurysm treatment has produced multiple options in treatment devices, each with individual considerations for follow-up imaging techniques and interpretation.
- •
Imaging for treated aneurysms should guide decision making for further follow-up with imaging or the need for reintervention.
Introduction
Intracranial aneurysms typically are saccular in morphology and located most often in the proximal anterior circulation at artery branch points around the circle of Willis. The prevalence of brain aneurysms in the adult population is estimated at approximately 2.3%; however, this figure varies depending on the evaluation method and population studied. , Rupture of an aneurysm with resulting intracranial hemorrhage (typically subarachnoid in location) is a rare but devastating event that carries a high risk of resulting neurologic morbidity and mortality, despite modern medical care. Although the prevalence of cerebral aneurysms in the general population is relatively high, routine screening generally is not performed, except in patients with a strong family history or other genetic predisposition, such as autosomal dominant polycystic kidney disease.
Most intracranial aneurysms are detected after acute subarachnoid hemorrhage; however, many are asymptomatic and detected incidentally on imaging studies performed for other indications. Management of such lesions is challenging, with the decision to treat dependent on multiple patient and lesion factors that are associated with a greater risk of future rupture. These include female gender, history of smoking or cocaine use, lesion size greater than 10 mm, and high aneurysm height/volume–to–neck width ratio as well as location in the posterior circulation. In contradistinction, aneurysms presenting following rupture carry a significant risk of recurrent hemorrhage, up to 20% in the first 2 weeks after presentation. Because each subsequent bleeding event increases the risk of patient morbidity and mortality, such lesions are treated promptly, if technically feasible.
Intracranial aneurysms may be treated via surgical or endovascular techniques. Surgical treatment involves craniotomy and placement of a titanium clip across the aneurysm neck. Prior to the widespread use of clips, aneurysm wrapping—with muscle, gauze, or other synthetic materials—routinely was performed; however, this procedure fell out of favor with the advent of surgical clipping. More recently, endovascular techniques have become widely utilized, including coil embolization, stent-assisted coiling, flow diversion, and intrasaccular flow disruption. Surveillance imaging typically is performed after aneurysm treatment to evaluate for lesion recurrence as well as to assess the patency of parent and branch vessels. This is true especially for aneurysms treated by some endovascular techniques, such as coiling, which carries a higher risk of lesion recurrence compared with surgical clipping (up to 20% for coiled aneurysms). ,
General considerations for follow-up of treated intracranial aneurysms
Follow-up imaging of treated intracranial aneurysms generally is performed using a variety of imaging modalities, including minimally invasive digital subtraction angiography (DSA), as well as various noninvasive techniques, such as MR angiography (MRA) and computed tomography angiography (CTA). DSA remains the gold standard for intracranial aneurysm imaging (treated or otherwise), due to its high spatial resolution, dynamic flow visualization, and sensitivity for aneurysm recurrence. , , DSA is more expensive and time-consuming, however, than noninvasive imaging modalities and carries risks to the patient both from the procedure itself and higher radiation exposure. Therefore, optimal follow-up imaging of treated aneurysms requires a multimodality approach tailored to the type of treatment. Suggested primary and alternative follow-up imaging modalities for each method of aneurysm treatment are summarized in Table 1 .
| Method of Treatment | Primary Follow-up Imaging Modality | Alternative/Supplemental Follow-up Imaging Modality |
|---|---|---|
| Surgical clipping | CTA | DSA for initial postoperative imaging or if evidence of recurrence |
| Coiling | CE-MRA | DSA if change or evidence of recurrence |
| Stent-assisted coiling | CE-MRA | DSA if change or evidence of recurrence CTA in select cases (evaluating stent patency) |
| Flow diversion | DSA CE-MRA | CTA with MAR DSA if change or evidence of recurrence |
| WEB device | DSA CTA | MRA DSA if change or evidence of recurrence |
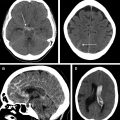
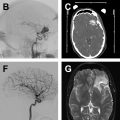
Comparison of the appearance of treated aneurysms on imaging can be difficult across modalities; however, using a simplified grading scheme can help improve standardization of reporting. The most widely used scale for grading aneurysm occlusion after treatment is that described by Raymond and colleagues, wherein class 1 denotes complete occlusion, class 2 denotes filling of the aneurysm neck ( Fig. 1 ), and class 3 denotes filling of the aneurysm sac. Other factors also must be accounted for when evaluating treated aneurysms, most importantly the patency of the parent vessel or branch vessels, position and configuration of an occlusion device, and patency of any stents, if present.
The exact follow-up imaging schedule after intracranial aneurysm treatment is dependent on several factors, including aneurysm size and configuration, prior rupture status, patient life expectancy, and the ability/desire of the patient to return for imaging. A general algorithm proposed by Soize and colleagues focusses on the Raymond occlusion grade to guide follow-up frequency. For stable class 1 or class 2 (adequately occluded) aneurysms, follow-up is performed at 3 months to 6 months, 6 months to 12 months, and then every 3 months to 5 years. If a neck remnant appears (class 1 to class 2) or enlarges, closer yearly follow-up should be performed for 5 years and then every 3 months to 5 years if stable. If an aneurysm remnant develops, DSA should be performed to evaluate for possible retreatment.
Surgically repaired aneurysms
Microsurgical repair of intracranial aneurysms has been an available treatment option since the early twentieth century. The technique consists of surgical dissection via a craniotomy to the target lesion followed by placement of 1 or more a titanium clip(s) across the aneurysm neck. The feasibility of aneurysm clipping is dependent on multiple factors. These include the location and configuration of the aneurysm, neck size, and presence of branch arteries.
Clipped aneurysms are followed primarily with CTA and/or DSA with 3-dimensional (3-D) rotational angiogram (RA). In 2006, Dehdashti and colleagues found CTA to be accurate and sufficient for follow-up of both excluded aneurysms and parent vessels compared with DSA, with artifacts significant enough to interfere with interpretation being rare (5%). Sensitivity and specificity for aneurysm remnants in that study both were reported at 100%; however, two 2-mm neck remnants were missed on prospective review and later identified after review with the treating surgeon. A more recent comparison of CTA and DSA post–aneurysm clipping showed a sensitivity of 83% for CTA in detecting recurrent aneurysms compared with 3-D RA as the gold standard, with all missed recurrences less than 2 mm. In cases of highly suspected recurrence or when CTA imaging is inadequate or nondiagnostic, DSA with 3-D RA can be performed to evaluate the need for retreatment further. MRA is not used frequently because the high degree of susceptibility artifact generated by the titanium clips precludes evaluation of the aneurysm sac and parent vessel.
Computed tomography (CT) studies can be limited significantly by beam-hardening artifact related to metallic aneurysm clips, a problem that decreased with the widespread use of titanium clips, which produce less artifact compared with other materials, such as cobalt alloy. , Newer CT technologies, including dual energy, spectral imaging, and metal artifact reduction (MAR), also have improved image quality markedly. Dual-energy and spectral imaging technology have become more widely available, allowing for image acquisition at multiple energies simultaneously and creation of virtual monoenergetic images across a range of energies. , Use of higher energies reduces artifact associated with clips; however, this must be balanced against loss of tissue contrast at higher energies.
When combined with MAR postprocessing algorithms, artifact can be reduced even further. In particular, newer iterative MAR algorithms can reduce artifact drastically, allowing for better visualization of adjacent vessels. In particular, use of MAR allows use of lower-energy imaging with dual-energy systems, improving signal-to-noise and contrast-to-noise while maintaining low levels of metal artifact. The introduction of novel artifacts has been described with MAR techniques, however, including false-positive vessel stenosis or occlusion, and these corrected images should be interpreted with care. , Virtual monoenergetic images with energies of 40 keV to 95 keV have been suggested as an appropriate balance between artifact reduction and visualization of vessel contrast, the lower-energy ranges performing better when used in conjunction with MAR. , ,
Coil embolized aneurysms
Aneurysm coiling has become an important endovascular alternative for treatment of intracranial aneurysms, showing lower rates of death or functional dependency on long-term follow-up compared with surgical clipping (at 10 years, 83% of patients were alive and 82% independent after coiling vs 79% and 78% after clipping, respectively). Coiling also is associated, however, with higher rates of recurrence and rebleeding compared with clipping, although the risk is small (1.56 rebleeds/1000 patient years after coiling; 0.49 rebleeds/1000 patient years after clipping). Recurrence after coiling may be related to multiple factors, including coil compaction, migration of the coil mass, enlargement of an aneurysm sac around the coil, and development of a new outpouching from the coiled aneurysm sac. Given the risk of recurrence, regular imaging follow-up of coiled aneurysms is standard of care.
Platinum coils generate significant beam-hardening artifact on CTA, most often precluding adequate visualization of the aneurysm sac as well as adjacent parent or branch vessels (see Fig. 1 ). The high density of the coils also can hinder visualization of recurrent/residual lesion filling on DSA, the gold standard examination, because central, interstitial filling within the coil mass may be obscured by the so-called helmet effect on 2-dimensional (2-D) angiograms. , This effect may be mitigated partially with the use of 3-D RA technique. Platinum coils meanwhile produce very little local magnetic field disruption and, therefore, little susceptibility artifact. MRA, therefore, is the preferred routine noninvasive imaging technique for follow-up, demonstrating high sensitivity for residual or recurrent aneurysm flow, including interstitial filling.
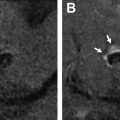
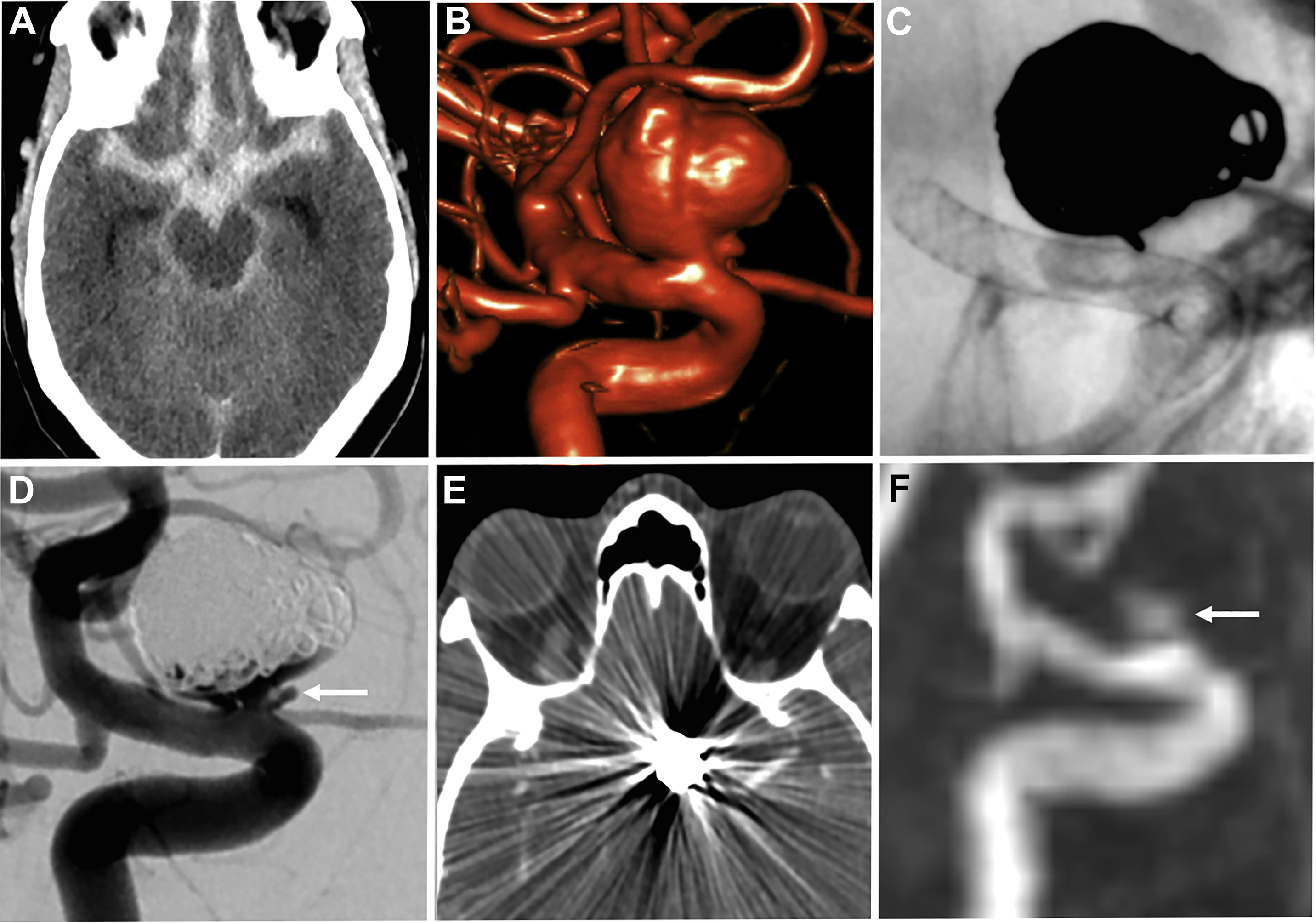
Both noncontrast time-of-flight (TOF) MRA and contrast-enhanced (CE) MRA are used routinely in the evaluation of aneurysms, each providing relative advantages and disadvantages. TOF-MRA is a T1-weighted technique that gives high spatial resolution without the need for intravenous contrast administration. The modality may suffer from several issues, however, including motion artifact due to longer acquisition times, eddy currents within the coil mass, or intra-aneurysmal signal loss from spin saturation and dephasing when slow or complex flow is present, as can occur in large lesions. , CE-MRA may help mitigate some of the artifacts from slow or turbulent blood flow associated with noncontrast technique. The modality has its own potential challenges, however, including venous contamination as well as enhancement of clot or vasa vasorum, which may be mistaken for flow-related enhancement. Finally, both TOF-MRA and CE-MRA are T1-weighted acquisitions, and incompletely suppressed T1 hyperintense material, such as acute thrombus, may be interpreted incorrectly as residual blood flow ( Fig. 2 ), although the use of subtraction imaging in CE-MRA as well as review of other sequences (T2-weighted) and multiplanar reformats may help prevent this error.
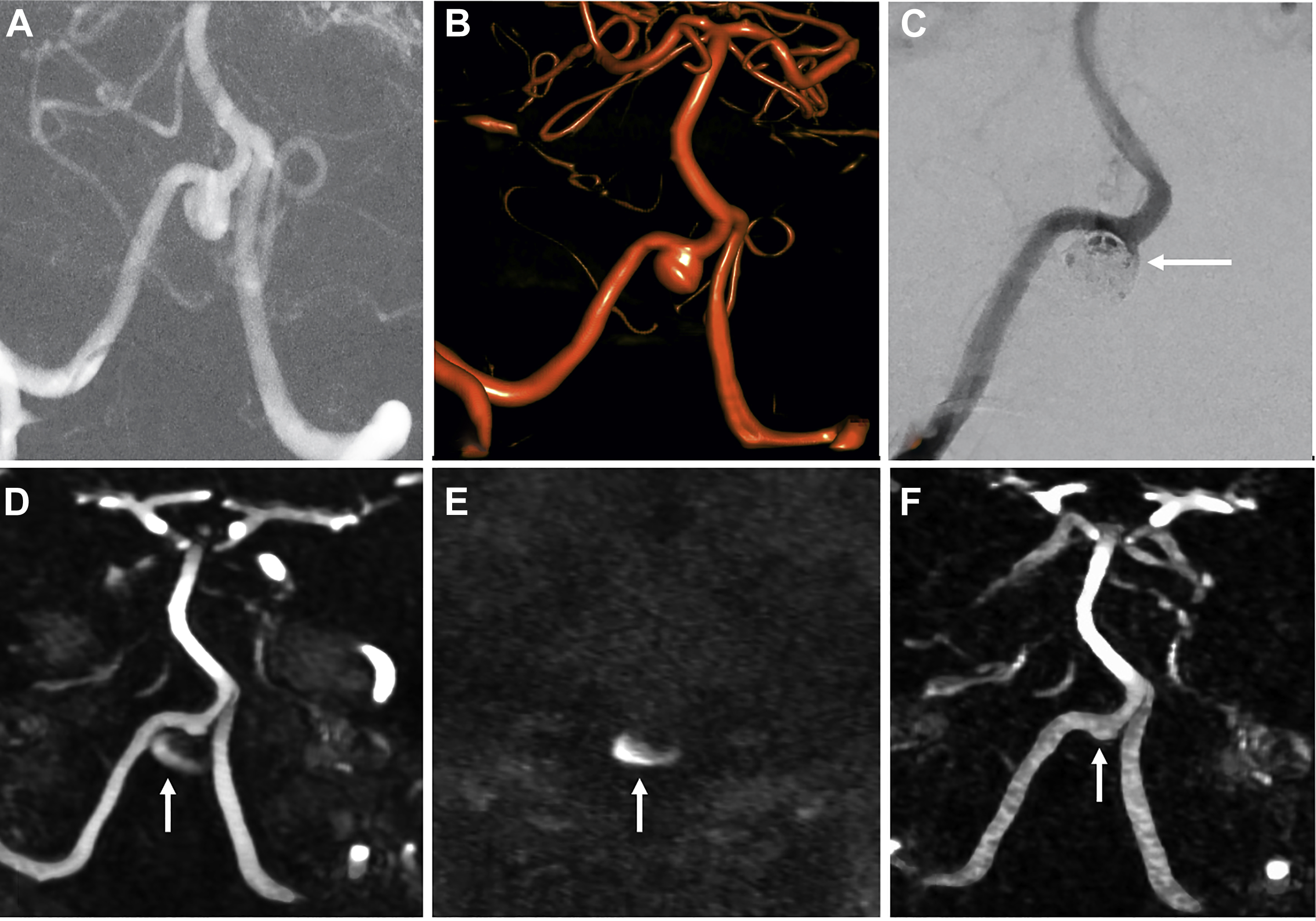
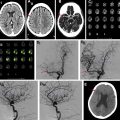
Sensitivity and specificity for residual filling of aneurysms have been shown to be quite high using either TOF-MRA or CE-MRA, with a recent meta-analysis showing sensitivity and specificity of 86% and 95%, respectively, for TOF-MRA, and 90% and 92%, respectively, for CE-MRA. , , Higher field strengths also are associated with less artifact related to the platinum coils, due to better spatial resolution and higher signal-to-noise ratio. , At the authors’ institution, TOF-MRA and CE-MRA both are performed for all coiled aneurysm follow-up studies, which the authors believe provide the best features of each examination type ( Fig. 3 ).
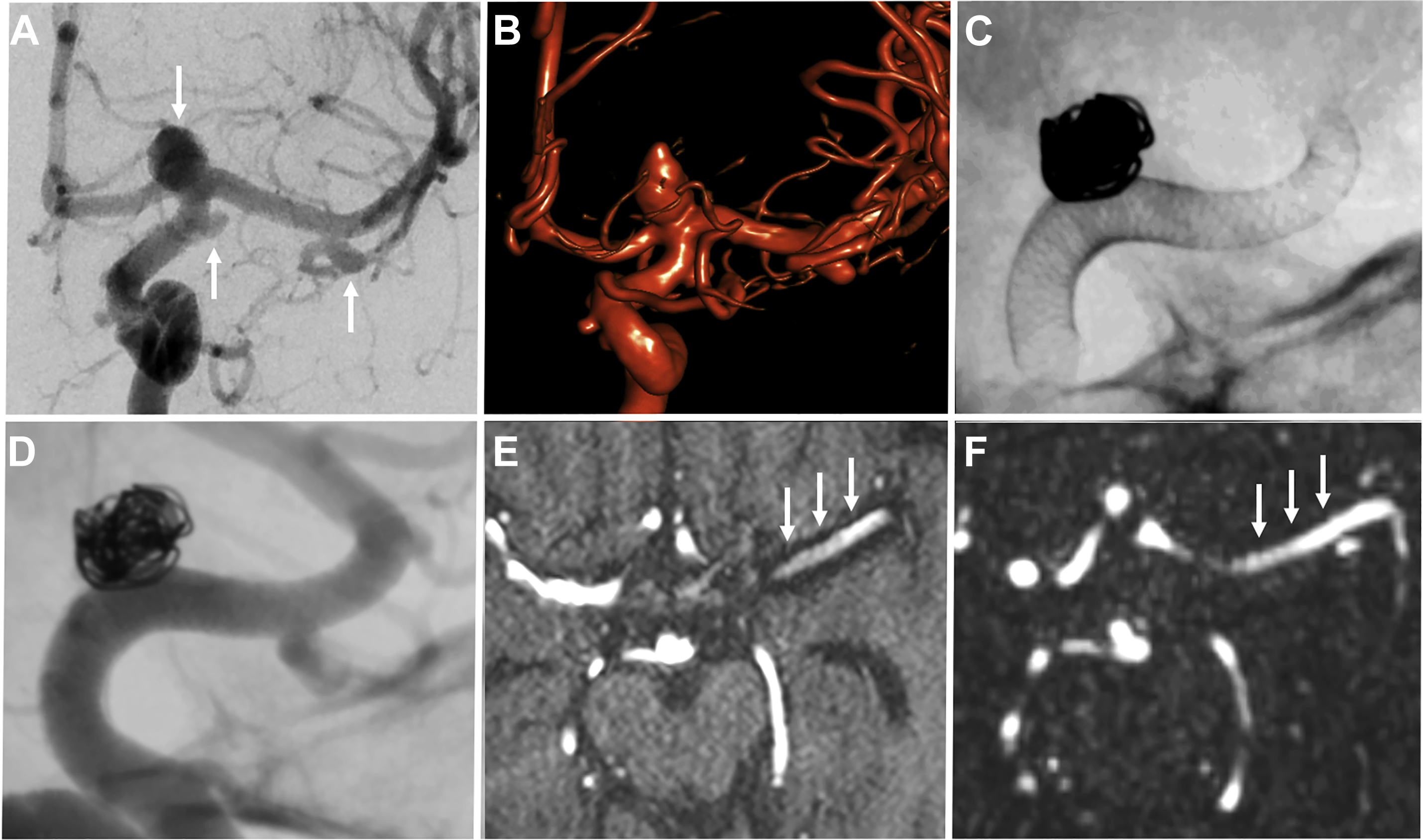
Stent-coiled aneurysms
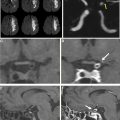
Stent-assisted coiling is a minimally invasive endovascular treatment of intracranial aneurysms wherein a bare metal stent is placed in the parent vessel across the aneurysm neck followed by coil placement in the target lesion. The stent serves to prevent coil prolapse or migration and is of particular use in wide-necked or bifurcation aneurysms ( Fig. 4 ). Dual stents in a Y-configuration also can be placed for coiling of bifurcation aneurysms, such as those at the basilar tip.