In this article the individual components of multimodal computed tomography and multimodal magnetic resonance imaging are discussed, the current status of neuroimaging for the evaluation of the acute ischemic stroke is presented, and the potential role of a combined multimodal stroke protocol is addressed.
Stroke is the third leading cause of death in the United States, Canada, Europe, and Japan. The American Heart Association and the American Stroke Association estimate that approximately 800,000 new strokes occur each year, resulting in more than 130,000 annual deaths in the United States alone. Direct and indirect costs related to stroke are estimated to be $70 billion annually, and will likely increase in the next decades. Stroke is the leading cause of adult disability in North America.
Ischemic stroke is caused by a reduction in the blood supply to the brain (usually a clot occluding a cerebral artery), which subsequently disrupts the supply of oxygen and nutrients to brain tissue. Ischemic strokes account for more than 80% of strokes and can be further subdivided into cardiogenic, atherosclerotic, lacunar, hemodynamic, or cryptogenic sources. Another 15% of strokes are related to the disruption of a cerebral artery, resulting in intracerebral hemorrhage (ICH). Other rare causes of stroke-like symptoms include subarachnoid hemorrhage, cerebral venous sinus thrombosis, chronic subdural hematoma, neoplasms, inflammatory disease, migraine, reversible cerebral vasoconstriction syndrome, seizure, and hypoglycemia. Some of these subjects are discussed in other articles elsewhere in this issue.
Imaging has revolutionized acute ischemic stroke diagnosis and management. Previously, structural imaging modalities, typically noncontrast computed tomography (NCT), were used to assess the presence and extent of acute ischemic stroke and exclude stroke mimics. With the development of functional imaging modalities such as perfusion CT (PCT) and perfusion magnetic resonance (MR) imaging, stroke has been redefined from an all-or-none to a dynamic and evolving process. In particular, the advent of effective thrombolytic therapies, such as intravenous tissue plasminogen activator (tPA), has motivated us to better define the so-called ischemic penumbra and improve patient selection for reperfusion therapy. Studies have indicated favorable clinical outcomes with thrombolytic therapies administered to patients selected by imaging criteria in an extended time window.
In this article, the individual components of multimodal CT and multimodal MR imaging are discussed, the current status of neuroimaging for the evaluation of the acute ischemic stroke is presented, and the potential role of a combined multimodal stroke protocol is addressed.
Physiopathology: the concept of penumbra
When a cerebral artery is occluded, a core of brain tissue dies rapidly. Surrounding this infarct core is an area of brain that is hypoperfused, but still viable because of collateral blood flow. This area of at-risk, but potentially salvageable, tissue is called the ischemic penumbra.
Studies in primates and positron emission tomography studies in humans have shown that brain parenchyma can compensate for hypoperfusion through an increase in oxygen extraction down to a cerebral blood flow (CBF) threshold of approximately 20 to 23 mL/100 g tissue/min. If the CBF decreases below this threshold, neuronal function is impaired. The affected neurons remain viable and recover without injury after normalization of the CBF as long as it remains more than approximately 10 to 15 mL/100 g tissue/min. If the CBF decreases below this point, a shortage of metabolites occurs, causing an Na + /K + channel failure in the ischemic cells. This membrane channel failure results in an uncontrolled net shift of extracellular water in the intracellular space. The consequence is cytotoxic edema and irreversible damage to the neuronal cells. The extent and severity of the CBF reduction and the neuronal integrity determine the size of the core and the penumbra.
The rate of change in the size of the core and penumbra is a dynamic process that depends mainly on the reperfusion of the ischemic brain. If the occlusion is not removed, the infarct core may grow and progressively replace the penumbra. In early recanalization, either spontaneously or resulting from thrombolysis, the penumbra may be salvaged from infarction.
Why image a patient with acute stroke?
The central premise of acute stroke treatment is to rescue the ischemic penumbra. The current guidelines neglect the fact that the portion of potentially salvageable ischemic tissue is not only dependent on the time window, but also on the individual patient’s collateral blood flow. The presence and extent of the ischemic penumbra are time-dependent , but are especially patient-dependent . From patient to patient, survival of the penumbra can vary from less than 3 hours to well beyond 48 hours. Of patients with supratentorial arterial occlusion, 90% to 100% show ischemic penumbra in the first 3 hours of a stroke, but 75% to 80% of patients still have penumbral tissue at 6 hours after stroke onset.
The only drug treatment of acute stroke approved by the US Food and Drug Administration is intravenous thrombolysis with recombinant tPA. It is limited to the first 3 to 4.5 hours after symptom onset, and after intracranial hemorrhage has been ruled out by NCT or gradient echo MR imaging. Only 3% to 8.5% of potentially eligible patients are treated because few are medically evaluated at an early enough stage, and there is the widespread concern of hemorrhagic conversion resulting from overly aggressive reperfusion therapy. However, it has been suggested that intravenous thrombolytic therapy can be safely administered beyond 3 to 4.5 hours in selected patients with a sufficient amount of salvageable brain tissue, thereby affording a safe treatment to a larger percentage of patients with stroke. This situation strongly emphasizes the need for an improved delineation of the salvageable ischemic penumbra by imaging in patients with acute stroke.
The negative results of thrombolysis trials between 3 to 6 hours may be because no method of penumbral imaging was used to select patients for therapy, despite penumbra being the target for treatment and despite the high percentage of patients with penumbra within this time window. Please see the article Acute Neuro-Interventional Therapies elsewhere in this issue for additional information regarding neurointerventional therapy for acute ischemic infarction.
A tissue clock that determines the presence and relative extent of both infarct and penumbra would be an ideal guide to patient selection for thrombolysis, rather than a rigid time window as is recommended by the current guidelines. During the last decade, several models for ischemic penumbra delineation using MR imaging, and increasingly, CT, have been developed to predict the outcome of ischemic brain tissue.
Why image a patient with acute stroke?
The central premise of acute stroke treatment is to rescue the ischemic penumbra. The current guidelines neglect the fact that the portion of potentially salvageable ischemic tissue is not only dependent on the time window, but also on the individual patient’s collateral blood flow. The presence and extent of the ischemic penumbra are time-dependent , but are especially patient-dependent . From patient to patient, survival of the penumbra can vary from less than 3 hours to well beyond 48 hours. Of patients with supratentorial arterial occlusion, 90% to 100% show ischemic penumbra in the first 3 hours of a stroke, but 75% to 80% of patients still have penumbral tissue at 6 hours after stroke onset.
The only drug treatment of acute stroke approved by the US Food and Drug Administration is intravenous thrombolysis with recombinant tPA. It is limited to the first 3 to 4.5 hours after symptom onset, and after intracranial hemorrhage has been ruled out by NCT or gradient echo MR imaging. Only 3% to 8.5% of potentially eligible patients are treated because few are medically evaluated at an early enough stage, and there is the widespread concern of hemorrhagic conversion resulting from overly aggressive reperfusion therapy. However, it has been suggested that intravenous thrombolytic therapy can be safely administered beyond 3 to 4.5 hours in selected patients with a sufficient amount of salvageable brain tissue, thereby affording a safe treatment to a larger percentage of patients with stroke. This situation strongly emphasizes the need for an improved delineation of the salvageable ischemic penumbra by imaging in patients with acute stroke.
The negative results of thrombolysis trials between 3 to 6 hours may be because no method of penumbral imaging was used to select patients for therapy, despite penumbra being the target for treatment and despite the high percentage of patients with penumbra within this time window. Please see the article Acute Neuro-Interventional Therapies elsewhere in this issue for additional information regarding neurointerventional therapy for acute ischemic infarction.
A tissue clock that determines the presence and relative extent of both infarct and penumbra would be an ideal guide to patient selection for thrombolysis, rather than a rigid time window as is recommended by the current guidelines. During the last decade, several models for ischemic penumbra delineation using MR imaging, and increasingly, CT, have been developed to predict the outcome of ischemic brain tissue.
Acute stroke MR imaging
Multimodal MR Imaging Stroke Protocol
A typical stroke MR imaging protocol consists of T2/fluid attenuated inversion recovery (FLAIR), T2*, diffusion-weighted (DW) and perfusion-weighted (PW) images ( Table 1 ) and MR angiography (MRA). This protocol can be performed in less than 30 minutes. It achieves reliable information about the site of vessel occlusion, the extent of potentially salvageable brain tissue, and the exclusion of differential diagnoses of ischemic stroke.
| Sequence | Single-shot Gradient Echo Echoplanar Imaging |
|---|---|
| Image acquisition parameters | TR ≤1500 ms TE = 35 to 45 ms at 1.5 T TE = 25 to 30 ms at 3 T Flip angle = 60° to 90° at 1.5 T Flip angle = 60° at 3 T |
| Image acquisition duration | 90 to 120 s Image acquisition started 10 s before initiation of bolus injection to achieve at least 10 baseline images |
| Coverage and slice thickness | Field of view ≈ 24 cm Whole brain coverage using ≥12 slices Gap 0 to 1 mm Slice thickness 5 mm Matrix size 128 × 128 |
| Slice orientation | Parallel to hard palate |
| Contrast material | Standard gadolinium-based contrast material |
| Contrast volume | Single dose (for half molar agent ≈ 20 mL for 100 kg person) followed by 20 to 40 mL saline flush |
| Injection rate | 4 to 6 mL/s; same rate for saline |
| IV access | 18- to 20-gauge IV line; right antecubital vein preferred |
The MR Imaging Protocol Sequence by Sequence
T2 and FLAIR imaging
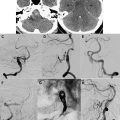
On T2-weighted and FLAIR images, ischemic infarction appears as a hyperintense lesion usually seen within the first 3 to 8 hours after stroke onset ( Fig. 1 ). In a recent study of patients with acute ischemic stroke studied by MR imaging within 6 hours of symptom onset, patients without a visible hyperintense lesion on FLAIR images had greater than 90% probability of being imaged within the first 3 hours of symptom onset. Thus, a mismatch between positive DW imaging and negative FLAIR images appears to be useful in the identification of patients who are likely to benefit from thrombolysis.
FLAIR images are also highly sensitive to subarachnoid hemorrhage as well as acute cerebral venous sinus thrombosis. In the setting of hyperacute stroke, T2-weighted images can be useful to detect the loss of the arterial signal flow void in occluded vessels within minutes of the stroke onset. T2-weighted and FLAIR images are both used to assess older cerebral infarctions and the extent of concomitant small vessel disease.
Diffusion-weighted imaging and apparent diffusion coefficient
Diffusion MR imaging provides image contrast that is dependent on the molecular motion of water. Cerebral ischemia leads to energy metabolism disruption with the failure of the Na + /K + and other ionic pumps. This situation induces a loss of ionic gradients and a net transfer of water from the extracellular to the intracellular compartment causing a cytotoxic edema. Excessive intracellular water accumulation leads to a reduced extracellular volume, which usually facilitates water mobility, and therefore to a reduction of water diffusion in the extracellular matrix. This phenomenon is detected with DW imaging within minutes of vessel occlusion and can be measured quantitatively with the apparent diffusion coefficient (ADC).
DW imaging is the most sensitive method for the depiction of ischemia in the hyperacute stage (see Fig. 1; Fig. 2 ). However, DW imaging lesions can be at least partially reversible in the early phase of ischemia, and the size of the DW imaging abnormality does not necessarily reflect irreversibly damaged tissue. A recent series of 68 acute patients with ischemic stroke showed that 20% of the patients had ADC normalization in greater than 5 mL of brain tissue. The partial normalization was predominantly seen in the basal ganglia and white matter in patients with distally located vessel occlusions, and it was associated with a trend toward a better clinical outcome. This study suggests that patients with a perfusion/diffusion match within 3 hours of symptom onset may still have salvageable tissue at risk and might benefit from thrombolysis.
Noncontrast MR angiography
The most commonly used noncontrast MRA technique is time-of-flight (TOF) imaging. This sequence depicts vascular flow by repeatedly applying a radiofrequency pulse to a volume of tissue, followed by dephasing and rephasing gradients. Stationary tissue in the volume becomes saturated by the repeated excitation pulses and has low signal. Conversely, inflowing blood protons are not saturated and therefore produce increased signal intensity. The vessel contrast is proportional to the blood velocity (ie, flow-related enhancement). For selective imaging of arteries, saturation bands are applied on the venous side of imaging sections to null signal from the venous flow.
Three-dimensional (3D) TOF-MRA is the preferred technique for the examination of intracranial vessels. MRA is particularly useful in the detection of vascular occlusion and/or stenosis in patients with ischemic stroke (see Fig. 2 ). Technical improvements such as parallel imaging and higher magnetic fields allow high spatial isotropic resolution, fast acquisition times, and reduced artifacts.
Perfusion-weighted MR imaging
PW imaging allows the measurement of capillary perfusion to the brain. The bolus passage of a paramagnetic intravascular MR imaging contrast agent through the cerebral capillaries causes a nonlinear signal loss on T2* images. This dynamic contrast-enhanced technique tracks the tissue signal changes caused by the susceptibility effect to create a hemodynamic time-to-signal intensity curve.
Gradient recalled echo (T2*) weighted imaging
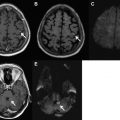
Hyperacute stroke imaging demands the differentiation between ischemic stroke and hemorrhagic stroke ( discussed in the article Hemorrhagic Stroke and Non-traumatic Intracranial Hemorrhage elsewhere in this issue ), which is impossible by clinical means only. Although CT is the standard method for the diagnosis of ICH, studies have shown that hyperacute ICH can be identified on MR (mainly FLAIR and gradient-recalled echo [GRE] imaging) with excellent accuracy.
Moreover, microbleeds (small hemosiderin deposits) not apparent on CT can be detected by T2*-weighted images. These chronic lesions are associated with an increased risk of spontaneous ICH and may also be a risk factor for thrombolysis-related hemorrhage.
In suspected acute stroke, T2*-weighted images can detect an intraluminal thrombus as a linear low signal region of magnetic susceptibility.
Alternative Sequences for Selected Conditions
Susceptibility-weighted imaging
Susceptibility-weighted (SW) imaging is a high-resolution 3D gradient echo sequence that uses magnitude and phase information to create a new source of contrast. It offers information about any tissue that has a different susceptibility than its surrounding structures, such as deoxygenated blood, hemosiderin, ferritin, and calcium.
SW imaging is exquisitely sensitive in detecting hemorrhage. Studies have shown that it is more sensitive in detecting hemorrhagic conversion and old microbleeds than CT and conventional GRE sequences.
Fresh clots contain a high concentration of deoxyhemoglobin, and thus appear hypointense on SW images. As a complementary sequence, SW imaging may be useful to depict distal branch thrombi that are not well visualized by MRA. Because of a decrease in arterial flow, and subsequent increase in the proportion of deoxyhemoglobin, acute thromboembolism may result in prominent hypointensity in the draining veins located in hypoperfused areas on SW imaging. In suspected acute stroke, therefore, SW imaging could also have a potential role by assessing areas of hypoperfusion without the need of contrast.
Despite its many potential advantages, this technique is not available on all major MR manufacturers’ systems and, despite the technical advances, approximately 5 minutes are still needed to image the entire brain.
Fat-saturated T1-weighted imaging
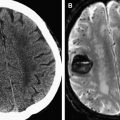
Fat-saturated T1 (T1-FS)-weighted images should be considered if cervical artery dissection is suspected. Vascular dissection is an important cause of acute infarction, causing up to 20% of cerebral infarctions in young patients. It occurs when blood extends into the wall of a vessel through an intimal tear.
Dissection occurs most frequently in the distal extracranial portion of the internal carotid artery and vertebral artery. It causes ischemic stroke primarily through embolization rather than through hemodynamic flow limitation. The intramural blood appears hyperintense on T1-FS-weighted images when met-hemoglobin develops, typically within 2 to 3 days after dissection ( Fig. 3 ).
Contrast-enhanced MRA of the cervical arteries
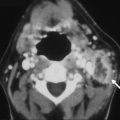
Contrast-enhanced MRA (CE-MRA) is the technique of choice for extracranial artery imaging. It relies on injection of a paramagnetic agent such as gadolinium to reduce the T1 relaxation time of tissue and to generate contrast between the intravascular lumen and surrounding tissues. Unlike TOF-MRA, vascular contrast is therefore relatively independent of flow dynamics, and artifacts associated with saturation effects are substantially reduced. Vessels from the aortic arch to the circle of Willis can be obtained in less than 1 minute. This sequence enables potential assessment of stenosis or vessel occlusion. CE-MRA is also used to show luminal narrowing in acute dissection (see Fig. 3 ).
MR Imaging for the Differentiation of Infarct Core and Penumbra
It has been hypothesized that DW imaging reflects the irreversibly damaged infarct, whereas PW imaging reflects the overall area of hypoperfusion. The volume difference between these techniques, termed the PW/DW imaging mismatch , represents the MR imaging correlate of the ischemic penumbra (see Figs. 1 and 2 ). Conversely, if there is no difference in PW and DW imaging volumes, or even a negative difference (PW < DW imaging), this is termed a PW/DW imaging match . This matched defect is seen in the patient who does not have penumbral tissue either because of normalization of previous hypoperfusion or because of completion of the infarction and the total loss of penumbra. It may be argued that this model does not take into account that the PW imaging lesion also assesses areas of oligemia that are not in danger and that DW imaging abnormalities do not necessarily turn into infarction. It is not yet clear which parameter gives the best approximation to critical hypoperfusion and allows differentiation of infarct from penumbra. However, most investigators agree that in current clinical practice, T max and mean transit time (MTT) seem to give the best results.
Stroke MR Imaging in Clinical Trials
Stroke MR imaging has been investigated in the clinical setting to evaluate its role for thrombolysis in an extended time window. The DIAS (Desmoteplase in Acute Ischemic Stroke) and DEDAS (Dose Escalation of Desmoteplase for Acute Ischemic Stroke) trials used a new fibrinolytic drug, desmoteplase, in patients with acute ischemic stroke within a 3- to 9-hour time window after symptom onset. Patient screening was based on clinical examination and medical history and guided by stroke MR imaging. Only patients showing a clear DW/PW imaging mismatch were randomized. The patients who received a placebo or an ineffective dosage showed a lower recanalization rate and an unfavorable outcome. Patients who achieved early vessel recanalization and reperfusion of penumbral tissue showed a significant clinical benefit, and 60% of the patients from the most effective dose tier had an excellent clinical outcome.
In the DIAS 2 study, patients were enrolled based on a mismatch diagnosed either by MR imaging or by PCT. The intention-to-treat analysis found no significant difference between the groups in clinical response rates, contrasting with their previous findings with desmoteplase in the DIAS and DEDAS trials. Clinical response rate was 46.0% in the placebo group, 47.4% in the group who received 90 μg/kg, and 36.4% in the group who received 125 μg/kg.
The DEFUSE trial was designed to determine whether a mismatch between perfusion and diffusion could be used to predict clinical outcome in patients with early reperfusion after treatment with recombinant tPA during the 3- to 6-hour time window after symptom onset. Patients with a baseline mismatch between PW and DW imaging of at least 20% and a reduction in perfusion abnormality volume of at least 10 mL had a better clinical outcome. The trial thus showed that baseline MR imaging findings can be used to identify groups of patients who are more likely to benefit from thrombolytic therapy and, potentially, other forms of reperfusion therapy. Data from this study suggested that a larger difference, a mismatch ratio (PW imaging volume-DW imaging volume/DW imaging volume) of 2.6, provided the highest sensitivity and specificity for identifying patients in whom reperfusion was associated with a favorable response. However, even in the presence of a large PW/DW imaging mismatch, no benefit could be expected if early recanalization of the occluded vessel failed.
Other trials have studied the role of early MR imaging changes for the prediction of thrombolysis outcome. Large ischemic lesions on DW imaging are predictive of poor outcome regardless of whether thrombolysis is performed. A prospective study in patients with anterior circulation ischemic stroke treated with tPA within 3 hours of stroke onset compared the baseline DW imaging findings using the Alberta Stroke Program Early CT Score (ASPECTS) with patient clinical outcome at 7 days. Clinical worsening and poor outcome were noted more frequently in patients with ASPECTS 5 or less. The investigators suggested that these patients should be excluded from studies of thrombolysis beyond the 3-hour time window.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree