Pelvic pain in the first trimester is nonspecific, with causes including pregnancy complications, pregnancy loss, and abnormal implantation, and symptom severity ranges from mild to catastrophic. Ultrasonography is the imaging modality of choice and essential to evaluate for the location of pregnancy, either intrauterine or not. If there is an intrauterine pregnancy, ultrasonography helps assess viability. If there is not an intrauterine pregnancy, ultrasonography helps assess for abnormal implantation, which accounts for a high percentage of maternal morbidity and mortality.
Key points
- •
In first-trimester pregnant patients, pelvic pain from pregnancy complications, pregnancy loss, or abnormal implantation is nonspecific, with symptoms ranging from mild to catastrophic, and ultrasonography should be performed early for better evaluation.
- •
On ultrasonography, first assess for the presence or absence of an intrauterine pregnancy (IUP). If there is an IUP, then assess for viability; if there is not an IUP, search for abnormal implantation.
- •
Abnormal implantation is seen in a small percentage of pregnancies; however, it accounts for a disproportionately high percentage of maternal morbidity and mortality.
Video content accompanies this article at http://www.radiologic.theclinics.com .
Introduction
In the first trimester, patient symptoms associated with pregnancy complications, pregnancy loss, and abnormal implantation are nonspecific. Most often, patients present to their providers or the emergency room with some symptom combination of pain and bleeding. Symptom severity ranges from mild to catastrophic. Ultrasonography is almost always the first modality used to image symptomatic early pregnant patients. This article begins by addressing diagnoses encountered when an intrauterine pregnancy is present because that is the first important diagnosis to make when imaging a pregnant patient with pain. An update on viability parameters is presented. Next, this article discusses presentations, causes, findings, and management strategies for abnormally implanted pregnancies.
First-trimester viability
Early first-trimester pregnancy failure occurs in approximately 10% of patients, accounting for 80% of all pregnancy losses. Transvaginal ultrasonography (TVUS) and concentration of maternal serum beta unit of human chorionic gonadotropin (β-hCG) are key tests to assess for early pregnancy failure.
For a normal intrauterine pregnancy (IUP), developmental milestones include :
- •
Gestational sac (GS) at 5 weeks
- •
Yolk sac at 5.5 weeks
- •
Visible embryo at 6 weeks
In 2013, new guidelines for evaluating early pregnancy loss were created by the Society of Radiologists in Ultrasound (SRU) Multispecialty Panel ( Table 1 ) because of updated research showing that the previously used cutoff values for crown rump length (CRL) and mean sac diameter (MSD) lead to false-positive interpretations of early pregnancy loss. The goal of these guidelines is to eliminate false-positive interpretations, preventing inappropriate medical or surgical management of a potentially viable desired IUP.
- •
SRU guidelines for definitive diagnosis of pregnancy failure :
- ○
CRL at least 7 mm without cardiac activity
- ○
MSD at least 25 mm without embryo
- ○
| Crown Rump Length Without Heartbeat (mm) | Mean Sac Diameter Without Embryo (mm) | GS Without YS; Length of Time for Follow-up US Not Showing Embryo with Heart Beat (d) | GS with YS; Length of Time for Follow-up US Not Showing Embryo with Heart Beat (d) | β-hCG | a Additional Findings | |
| Diagnostic for Early Pregnancy Loss | >7 | >25 | 14 | >11 | No single threshold value; correlated with trend of values on surveillance | |
| Suspicious for Early Pregnancy Loss | <7 | 16–24 | 7–13 | 7–10 |
a Additional findings suspicious for, but not diagnostic of, early pregnancy loss: no embryo more than 6 weeks after LMP YS larger than 7 mm. Empty amnion: amnion adjacent to YS without embryo. Early oligohydramnios: less than 5 mm size difference between mean sac diameter and crown rump length.
More recent studies suggest limitations of the 2013 SRU guidelines, including the following from the 2018 American College of Obstetrics and Gynecology :
- •
Strict measurement and observational cutoffs may limit clinical management because of patient preferences
- ○
For example, willingness to wait for 100% certainty of failure
- ○
- •
Few data points near cutoff values in studies used for SRU guidelines
- •
SRU guidelines are more conservative than data in literature and lead to patient stress and anxiety
- ○
Literature: no embryo seen at 7-day follow-up of GS without embryo was 100% specific in a study of 359 patients
- ○
SRU guidelines recommend 14-day follow-up of GS without embryo
- ○
Historically, a “discriminatory level” of β-hCG was used for evaluation of IUP development. However, recent guidelines challenge the use of a discriminatory level:
- •
Absolute β-hCG level should not be used to determine when an IUP should be visualized ,
- ○
Potential for false-positive interpretation of early pregnancy loss
- ▪
Instead, use accurate gestational age
- ▪
- ○
- •
Historically: IUP seen on TVUS at β-hCG level of 1000 mIU/mL
- ○
However, follow-up IUP with cardiac activity has been seen when initial GS was empty and β-hCG level 2000 to 3000 mIU/mL
- ○
Multiple gestations have higher β-hCG levels than singleton at same gestational age
- ○
Updated use of β-hCG: more conservative value should be used (eg, >3500 mIU/mL) for potential ectopic
- ▪
Goal: prevent termination of normal IUP (ie, false-positive)
- ▪
In hemodynamically stable patients, follow-up ultrasonography and/or serial β-hCG are most helpful
- ▪
- ○
Intrauterine pregnancy and bleeding
Perigestational hemorrhage (PGH) occurs in asymptomatic and bleeding first-trimester patients. Various methods of analysis have been used, including subjective size determination, percentage surrounding the GS, and volumetric analysis, but these do not statistically correlate with chance of early pregnancy loss. Measuring the perigestational hemorrhage as a percentage fracture of the GS is most predictive of early pregnancy loss.
As the size of PGH increases as a percentage fraction of GS, the rate of demise increases.
- •
PGH size less than or equal to 10% of GS equals 5.8% demise
- •
PGH size greater than 50% of GS equals 23.3% demise ( Fig. 1 )

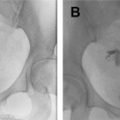
Fig. 1
Large perigestational hemorrhage and subsequent embryo demise. ( A ) The GS is barely attached to the uterus ( arrow ), surrounded by intrauterine blood ( open arrows ). ( B ) A living embryo was present. ( C ) On follow-up, there was embryo demise.
- •
Earlier diagnosis of PGH portends a worse outcome
- ○
Less than or equal to 8 weeks: 17.3% demise
- ○
Greater than 8 weeks: 3.6% demise
- ○
- •
PGH in patients greater than or equal to 35 years old equals higher loss than more than 35 years old
Intrauterine pregnancy and pain
Pelvic pain in the presence of an IUP adds several additional causes to the differential, including ovarian torsion (covered in a separate article in this issue), ruptured corpus luteum/hemorrhagic follicular cyst, and (rarely) red degeneration of a leiomyoma.
- •
Ruptured ovarian cyst (corpus luteum/hemorrhagic follicular cyst)
- ○
Often imaging overlaps between the two: similar presentations
- ○
Ultrasonography findings ( Fig. 2 , [CR] ):
- ▪
Ovarian lesion with complex internal echogenicities and increased through transmission; partially collapsed with thick, irregular wall
- ▪
Early hemorrhage: blood can be anechoic
- ▪
First 24 hours: lacelike internal echogenicities
- ▪
Later: retracting clot with angular, curvilinear echogenic material
- ▪
While resolving: fluid-debris level during clot liquefaction

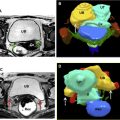
Fig. 2
Ruptured corpus luteum (CL) as cause of hemoperitoneum. ( A ) Transabdominal sagittal view of the uterus shows an IUP ( arrow ). However, the uterus is surrounded by fluid ( open arrows ). ( B ) Transvaginal ultrasonography of the right ovary ( arrows ) shows classic ring-of-fire color Doppler finding of a CL. ( C ) Adjacent to the ovary, there is a large heterogeneous masslike structure without flow, a blood clot in the adnexa. Subsequently, imaging of the upper abdomen was performed. ( D ) The hemorrhage extends to the right upper quadrant. Arrows point to blood anterior to the kidney.
- ▪
- ○
Computed tomography
- ▪
Intermediate-density to high-density ovarian lesion (25–100 HU)
- ▪
At rupture:
- •
May have internal fluid/blood level
- •
May be partially collapsed with irregular, thick wall
- •
Hemoperitoneum, which can be extensive
- •
- ▪
- ○
Tubal ectopic pregnancy
Ectopic pregnancies account for 2% of all pregnancies, and approximately 90% are in the fallopian tube. , The prevalence of ectopic pregnancy in women presenting to the emergency department with pelvic pain, bleeding, or both is near 20%. , , Severity of symptoms range from mild cramping and spotting to shock from internal hemorrhage. Ruptured ectopic pregnancy was the leading cause of hemorrhage-related mortality in pregnancy in 2011 to 2013.
Risk Factors
Increased risk for tubal ectopic pregnancy (TEP) is associated with damage to the fallopian tube, although half of patients have no identifiable risk factors. The following risk factors are associated with TEP , , :
- •
Prior pelvic inflammatory disease
- •
History of pelvic or tubal surgery
- •
Use of assisted reproductive technology (ART)
- •
History of prior TEP (10% chance for a second TEP, 25% chance of a third TEP)
- •
Indwelling intrauterine device (IUD) (53% incidence for TEP)
Imaging Technique
Transvaginal ultrasonography (TVUS) is the gold standard for imaging TEP. , However, all imaging should start with transabdominal ultrasonography (full bladder not required) in order to see whether there is a large mass or fluid extending into the abdomen, both of which can be missed with TVUS-only scanning.
Imaging protocols should include adequate documentation of the uterus and its contents, ovary, adnexa, and posterior cul-de-sac. Documentation of a dynamic examination using video clips/cine loops is recommended in order to show motion of structures in relation to each other. Probe pressure can aid in showing the relationship of adnexal masses to the ovary (ie, whether the mass moves with or separate from the ovary and uterus). External pressure with the nonscanning hand can be additive for this purpose as well.
Intrauterine Findings
The most reassuring sign that the patient does not have TEP is the presence of an IUP. However, uterine findings with TEP vary and include:
- •
Empty uterus with variable endometrial thickness
- ○
Thin empty endometrium
- ○
Diffusely echogenic endometrium from diffuse decidual reaction
- ○
Decidual cysts
- ▪
Thin wall without peripheral flow (can mimic early IUP)
- ▪
- ○
- •
Uterus with intracavitary blood
- ○
Diffusely distributed: endometrium distended by hypoechoic material
- ○
Focal collection: can mimic an IUP, so-called pseudosac
- ▪
More centrally located than normal IUP
- ▪
No yolk sac or embryo
- ▪
Might move within endometrium with probe pressure
- ▪
- ○
- •
Rarely, IUP can be seen along with TEP: heterotopic pregnancy ,
- ○
Incidence of 1% in patients who have undergone in vitro fertilization
- ▪
Otherwise, incidence is 1 in 4000 to 30,000
- ▪
- ○
Look for TEP in symptomatic in vitro fertilization patients, even if IUP present
- ○
The adnexal findings with TEP can be divided into those that lead to a definitive diagnosis and those suggestive of the diagnosis , , , :
- •
Adnexal findings definitive of TEP
- ○
GS with yolk sac plus or minus embryo in adnexa
- ▪
Separate from ovary
- ▪
- ○
Document embryo cardiac activity if present
- ○
- •
Adnexal findings suspicious for TEP (more common) ( Fig. 3 , [CR] )
- ○
GS without yolk sac/embryo in adnexa, separate from ovary
- ▪
Echogenic ringlike mass separate from ovary
- ▪
Use probe pressure to show GS and ovary move separately
- ▪
Find corpus luteum (CL) in ovary (often on same side as TEP)
- ▪
GS echogenic wall is almost always brighter than CL
- ▪
Color Doppler: ring-of-fire trophoblastic flow in TEP; however, CL may also have ring-of-fire peripheral flow appearance
- ▪
Pulse Doppler: high-velocity low-resistance flow in TEP, lower velocity and also low resistive flow in CL
- ▪
- ○
Nonspecific adnexal mass separate from ovary
- ▪
Hematoma within dilated tube: small round, or tubular morphology
- ▪
Ruptured tube with hematoma: large, any shape
- ▪
Most often without vascular flow, although color Doppler may show otherwise hidden GS in blood clot
- ▪
80% positive predictive value as an isolated finding
- ▪
- ○
Complex peritoneal fluid: pelvic fluid with echoes
- ▪
Small amount of fluid is first seen in posterior cul-de-sac
- •
Blood leaking from tube
- •
Angle probe and look immediately posterior to cervix
- •
- ▪
Look in abdomen if large amount of fluid is seen in pelvis or large adnexal hematoma
- •
Findings suggest ruptured TEP
- •
- ▪
- ○
Differential diagnosis of adnexal findings: mimickers of TEP
- ▪
CL: intact or ruptured
- •
More hypoechoic than TEP
- •
Hemorrhagic CL can contain echoes mimicking embryo
- •
Moves with the ovary, not separate from ovary
- •
When ruptured, can cause symptomatic hemoperitoneum
- •
- ▪
Paratubal cyst or hydrosalpinx
- •
Thin-walled, anechoic, no peripheral blood flow
- •
- ▪
Endometrioma
- •
Diffuse medium-level echoes, although decidualization with pregnancy causes heterogeneous appearance and internal flow
- •
- ▪

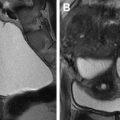
Fig. 3
Nonruptured TEP in patient with right adnexal pain. ( A ) Transvaginal ultrasonography shows a retroflexed uterus with thin endometrium ( calipers ) and surrounded by minimally complex pelvic fluid ( arrow ). ( B ) Adjacent to the right ovary there is an echogenic ring ( arrows ), the TEP (this shows the so-called sliding sign separate from the ovary). ( C ) Note that the tubal ectopic is more echogenic than the ovary and the CL ( arrows ). ( D ) Typical ring-of-fire peripheral flow is seen. This patient would have been a candidate for methotrexate (MTX) but opted for surgery. ( E ) Laparoscopic image shows a dilated intact fallopian tube ( arrow ) and a small amount of blood adherent to the right ovary ( thick arrow ). ( F ) The tube was incised and the intact GS ( arrows ) extracted.
- ○
Management and Treatment
TEP is a nonviable pregnancy and management decisions depend on patient clinical status, history, and imaging findings. The options for treatment and management include medical, surgical, and surveillance. ,
- •
Methotrexate (MTX) as medical treatment
- ○
MTX is a folate antagonist with cytotoxic effects on actively dividing cells (ie, trophoblasts)
- ○
Intramuscular injection is most common route
- ▪
Single-dose and multiple-dose protocols are described
- ▪
- ○
Higher success rates with earlier TEP diagnosis
- ▪
TEP less than 4 cm on ultrasonography
- ▪
No embryo cardiac activity
- ▪
hCG levels less than 5000 mIU/mL
- ▪
- ○
Relative contraindications
- ▪
Embryo cardiac activity
- ▪
hCG level greater than 5000 mIU/mL
- ▪
TEP greater than 4 cm
- ▪
Patient does not accept blood transfusion
- ▪
- ○
Absolute contraindications
- ▪
Heterotopic pregnancy/IUP
- ▪
Ruptured TEP
- ▪
Hemodynamically unstable patient
- ▪
Patient cannot participate in follow-up surveillance
- ▪
Medical reason for MTX intolerance (ie, hepatic or renal disease)
- ▪
- ○
Surveillance after MTX treatment
- ▪
hCG followed until nonpregnant levels
- •
hCG levels may initially increase but then progressively diminish
- •
Should regress by at least 15% by 4 to 7 days after start of treatment
- •
- ▪
Ultrasonography surveillance is not routine
- •
Imaging findings do not predict rupture or time to resolution
- •
Initial findings may show a larger TEP even when treatment is successful
- •
- ▪
- ○
- •
Surgical treatment are salpingostomy (tube sparing) and salpingectomy (removal of tube)
- ○
No significant difference in rates of subsequent IUP with 2 different techniques
- ○
Salpingectomy preferred if severe tubal damage seen at time of surgery
- ○
hCG surveillance after salpingostomy recommended because residual trophoblastic tissue might be present
- ▪
Prophylaxis with single-dose MTX after salpingostomy in some cases
- ▪
- ○
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree