Acute pelvic pain in the nonpregnant woman is one of the most common conditions requiring emergent medical evaluation in routine clinical practice. Although clinical evaluation and laboratory testing are essential, imaging plays a central role. Although various adnexal and uterine disorders may result in acute pelvic pain of gynecologic origin, other nongynecologic disorders of the gastrointestinal and genitourinary systems may likewise result in acute pelvic pain. Ultrasound is first choice for initial evaluation of acute pelvic pain of gynecologic origin. Computed tomography is performed if pelvic sonography is inconclusive, or if a suspected disorder is nongynecologic in origin.
Key points
- •
Pelvic pain is a common condition that affects women of all ages and is defined as acute when less than 3 months’ duration.
- •
The differential diagnosis of acute pelvic pain in the nonpregnant woman is broad, including a variety of gynecologic and nongynecologic entities.
- •
Ultrasound serves as the primary imaging modality for the evaluation of acute pelvic pain in the nonpregnant woman.
Introduction
Pelvic pain is a common condition that affects women of all ages and is defined as acute when less than 3 months in duration. The etiology of acute pelvic pain in women of reproductive age often poses a diagnostic dilemma because many signs and symptoms are nonspecific. In addition, the clinical presentation of each disorder can vary widely. A detailed history regarding onset, character and duration of pain, constitutional symptoms, relevant laboratory values, and gynecologic, sexual, and social history can aid in the diagnosis. In the nonpregnant woman, the differential diagnosis of acute pelvic pain is broad, including a variety of gynecologic and nongynecologic entities. Imaging plays a central role in the diagnosis and behooves the radiologist to become familiar with the most common etiologies.
Imaging protocols
Although ultrasound, computed tomography (CT), and magnetic resonance (MR) imaging are all used in the evaluation of acute pelvic pain in nonpregnant women, the selection of imaging modality for initial evaluation should be driven by the most clinically suspected disorder ( Box 1 ). However, as CT uses ionizing radiation, its use should be avoided in young women of reproductive age unless there is a clear risk-to-benefit ratio.
Gynecologic Etiology Suspected
- •
Transabdominal ultrasound (TAS), transvaginal ultrasound (TVS), and Doppler ultrasound
- •
MR imaging if ultrasound nondiagnostic or inconclusive
- •
Nongynecologic Etiology Suspected
- •
Computed tomography
- •
Ultrasound if appendicitis or urinary tract pathology is suspected; MR if ultrasound nondiagnostic or inconclusive
- •
Gynecologic etiologies
Multiple conditions of the female reproductive tract may account for acute pelvic pain in the nonpregnant woman of reproductive age ( Box 2 ). The following is a discussion of some of the more common etiologies.
Adnexal
- •
Functional cysts
- •
Hemorrhagic cysts
- •
Ovarian torsion
- •
Pelvic inflammatory disease
- •
Ovarian hyperstimulation syndrome
- •
Ovarian vein thrombophlebitis
- •
Uterine
- •
Malpositioned intrauterine device
- •
Leiomyoma degeneration/prolapse/torsion
- •
Adnexal disorders
Functional Cysts
Ovarian follicles are estrogen-mediated cystic lesions containing an oocyte that progressively enlarges during the first half of the menstrual cycle. One or more dominant follicles eventually emerge within the ovary ranging from 17 to 28 mm in diameter. At midcycle, the dominant follicle evolves into a corpus luteum that secretes hormones to prepare for implantation and support early pregnancy. A follicle that fails to expel an oocyte may continue to enlarge as a follicular cyst. Although most follicular cysts are asymptomatic, rapid cystic enlargement or rupture may result in acute pelvic pain. Follicular cysts generally measure between 3 and 10 cm and may remain hormonally sensitive. A discriminatory size of 3 cm also differentiates a physiologic corpus luteum from a corpus luteal cyst.
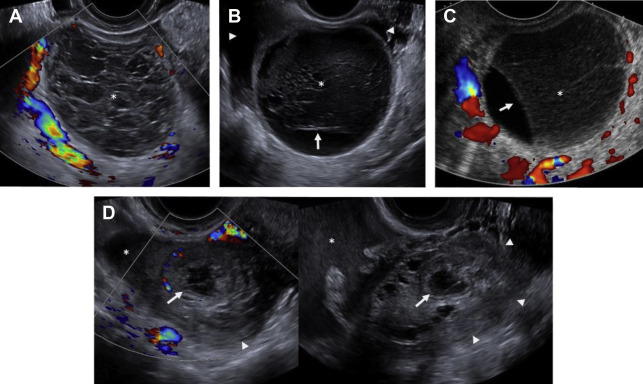
On ultrasound , follicular cysts are unilocular, round or oval, anechoic intraovarian lesions with posterior acoustic enhancement. The inner margin is uniformly smooth and a peripheral rim of compressed ovarian parenchyma is often seen. , Follicular cysts are typically sharply marginated, unilocular, thin-walled cysts with simple internal fluid attenuation on CT. On MR, follicular cysts are isointense to simple fluid on various pulse sequences: uniformly hypointense on T1-weighted images and uniformly hyperintense on T2-weighted images. The thin walls of follicular cysts are typically best appreciated on T2-weighted images, and may enhance on T1-weighted contrast-enhanced images ( Fig. 1 ).
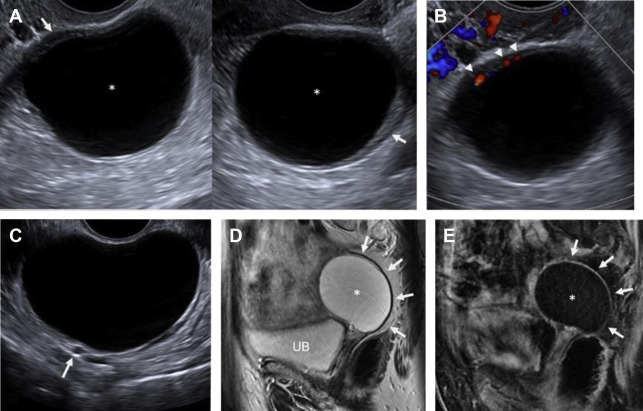
In contrast, on ultrasound , a corpus luteum has a thick wall, smooth or crenulated inner margin, and commonly demonstrates intense peripheral flow. The highly friable nature of its luteinized walls explains the common appearance of echoes within the central cystic component (see the next section, “Hemorrhagic cysts”). Corpus luteal cysts often demonstrate smooth or crenulated thickened walls with robust enhancement on CT and MR, but should never contain any discrete enhancing solid components ( Fig. 2 ).
Hemorrhagic Cysts
Hemorrhagic cysts result from intracystic bleeding into a follicle or corpus luteum. Although many hemorrhagic cysts are discovered incidentally, others present with severe pain due to rapid enlargement or rupture. Although hemorrhagic cysts are typically smaller than 5 cm, they may exceed 10 cm in size.
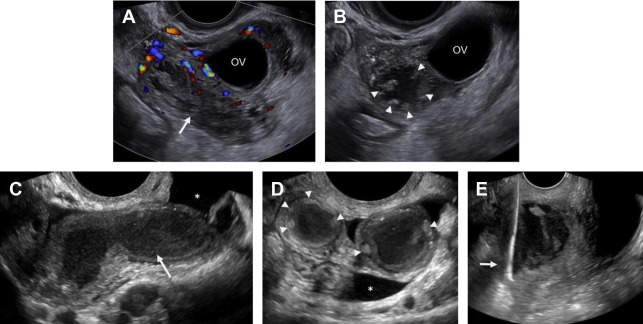
On ultrasound , hemorrhagic cysts are cystic ovarian lesions containing internal fibrin strands that are often described as reticular, lacelike, fishnet, or cobweb in appearance. , Retractile clot may demonstrate straight or concave margins, the latter of which has a 100% specificity for a benign hemorrhagic cyst (see Fig. 4 ). Peripheral vascularity is often seen on color Doppler interrogation. Acute rupture of a hemorrhagic cyst may reveal hematoma and/or hemoperitoneum within the adnexa or cul-de-sac ( Fig. 3 ).
On CT, hemorrhagic cysts demonstrate higher attenuation than that of simple fluid, typically greater than 30 Hounsfield units. On MR, these lesions frequently demonstrate internal signal intensity higher than that of simple fluid on T1-weighted images due to the presence of internal blood products, although the signal intensity may vary depending on the chronicity of the cyst. These lesions are typically intermediate to high signal intensity on T2-weighted images.
Ovarian Torsion
Ovarian torsion occurs when the ovary and accompanying support structures twist on its vascular pedicle. Predisposing risk factors include prior pelvic surgery, early pregnancy, ovulation induction medication, and intraovarian lesions in 50% to 80% of reported cases. , Lesions that tend to twist are often larger (>4–5 cm) and are much more commonly benign than those lesions due to malignancy, endometriosis, or infection, which “fix” the ovary in place. However, torsion can likewise occur in prepubertal girls with normal ovaries. Classic symptoms of ovarian torsion include sudden onset of sharp or stabbing pelvic pain. Less specific symptoms, such as pain-induced nausea and vomiting, are seen in 70% to 85% of women with torsion. The twisting of the ovarian vascular pedicle can compromise arterial, venous, and lymphatic flow to the affected ovary. As ovarian salvage rates are known to be inversely proportional to time to surgical intervention, imaging plays a critical role in prompt diagnosis.
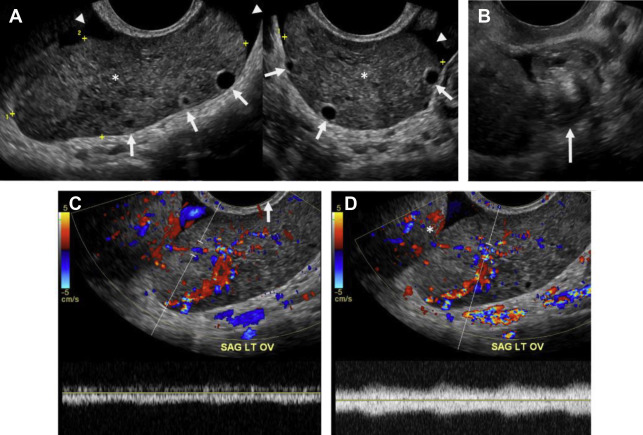
The classic ultrasound appearance of ovarian torsion is diffuse edema manifest as ovarian enlargement (5–20 cm) or increased surrounding ovarian parenchymal thickness when an intraovarian lesion is present. Other manifestations of edema include peripheralization of ovarian follicles (“string of pearls” sign) and adjacent free fluid. Additional grayscale findings of torsion include unusual ovarian location, uterine deviation/tilting and visualization of the twisted pedicle. ,
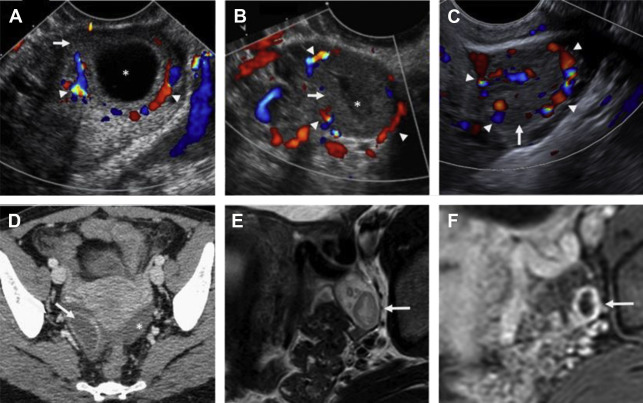
Although duplex Doppler interrogation of the ovary may aid in the diagnosis of torsion, the presence or absence of flow within the ovary or twisted pedicle may play a greater role in assessing ovarian viability. An absence of both arterial and venous flow is classically seen in high-grade torsion. However, arterial flow may be preserved in the setting of early or partial torsion in which only venous flow is compromised. Color Doppler evaluation may reveal a twisted or corkscrew appearance of the vascular pedicle, the so-called “whirlpool sign” ( Fig. 4 ). The presence of flow within the twisted pedicle by Doppler interrogation can aid in assessing the viability of the ovary in experienced hands. Most importantly, the presence of flow within the ovary does not exclude torsion and should never negate worrisome grayscale ultrasound findings when torsion is clinically suspected.
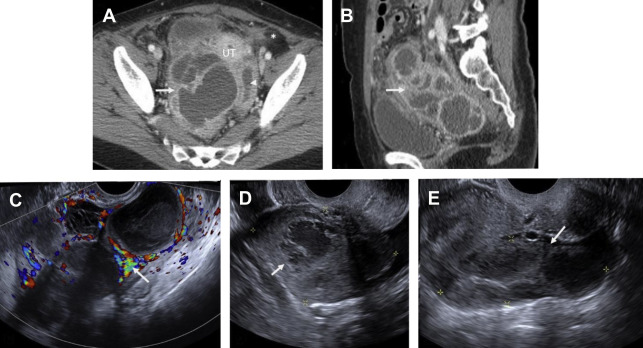
CT findings of torsion mirror those seen on ultrasound, including asymmetric ovarian enlargement, hypoattenuation, hypoenhancement, unusual situs, deviation/titling of the uterus toward the ipsilateral side, and stranding of adnexal fat when ischemia is present. The twisted pedicle sign may be seen on CT and is more frequently appreciated in the coronal plane ( Fig. 5 ).
Pelvic Inflammatory Disease
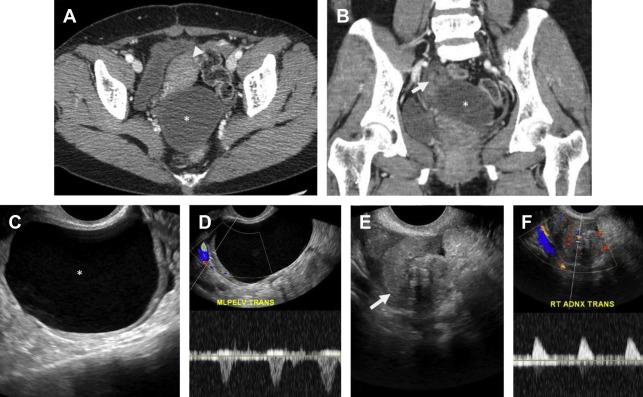
Pelvic inflammatory disease (PID) is a spectrum of infection of the upper female genital tract including endometrium, fallopian tubes, ovaries, and peritoneal cavity. PID is one of the most common causes of acute pelvic pain in sexually active women, affecting 1 million women and resulting in 275,000 hospitalizations per year. The most common pathogens in PID are Neisseria gonorrhea and Chlamydia trachomatis , although polymicrobial infection is implicated in 30% to 40% of cases. Infection typically spreads from the vagina when the cervical mucus plug is expelled during menses and spreads in ascending order to involve the uterus, tubes, and ovaries, often asymmetrically. Several discrete, often coexisting entities have been described, including cervicitis, endometritis, myometritis, salpingitis, pyosalpinx, tubo-ovarian complex (TOC), and tubo-ovarian abscess (TOA).
PID should be suspected in any female individual presenting with acute pelvic pain, fever, and leukocytosis. Untreated PID can eventually result in pyosalpinx and TOA, often requiring imaging-guided or surgical drainage. Severe PID may result in tubal scarring with increased incidence of infertility, ectopic pregnancy, and chronic pelvic pain. Although PID is considered a clinical diagnosis, imaging can greatly aid in the detection and guiding appropriate management of PID-related complications.
Pelvic ultrasound is frequently normal in early PID, although affected women may complain of cervical motion tenderness with transvaginal sonography. Sonographic features of PID-induced endometritis include endometrial thickening, heterogeneity, and hypervascularity and occasionally intracavitary fluid ± echoes. Increased vascularity within the myometrium may suggest endomyometritis. Echogenic fluid may develop in the pelvic cul-de-sac. Pyosalpinx may develop in the setting of salpingitis, which manifests as a thick-walled, hypervascular tubal-shaped structure between the uterus and ovary. A “cogwheel” sign may be present as thickened longitudinal folds of the fallopian tubes demonstrate a nodular appearance when seen in cross section ( Fig. 6 ). TOAs may develop in which the margins between tube and ovary are obscured, resulting in a complex, thick-walled cystic adnexal mass or tubo-ovarian complex. The term TOC is used by some as distinct from TOA when an adjacent edematous ovary is inseparable from the tubal process. On ultrasound, a TOA presents as a heterogeneous, complex cystic adnexal mass ± thickened walls, fluid-debris levels, and surrounding hypervascularity without an identifiable ovary ( Fig. 7 ).