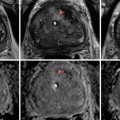
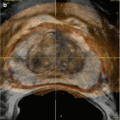
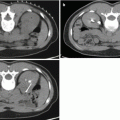
Fig. 5.1
Non-contrast CT scan, axial cut demonstrating enlarged prostate
Prostatitis
Prostatitis refers to inflammation of the prostate gland. Infection occurs when bacteria from the urethra or bladder enter the prostate. It has been reported that there are approximately 2 million outpatient visits yearly for prostatitis in the United States, accounting for 8 % of all urology office visits and 1 % of all primary care visits [23, 24].
The National Institutes of Health (NIH) established a classification of prostatitis into four categories: acute bacterial prostatitis, chronic bacterial prostatitis, chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), and asymptomatic inflammatory prostatitis. Acute bacterial prostatitis presents with dysuria, frequency, and other symptoms of a urinary tract infection. E. coli is the most common causative pathogen. Signs of systemic infection are related to infection of the prostate and include fever, malaise, and general aches. Treatment for acute bacterial prostatitis is directed at gram-negative enteric bacteria. Trimethoprim-sulfamethoxazole, fluoroquinolones, and ampicillin with gentamicin are all reasonable options while awaiting urine culture sensitivities and should continue for a total of 1 month duration. Additionally, alpha-adrenergic blockers can be used to help reduce obstruction and urinary reflux. Rarely, prostatitis requires surgery and this is generally seen in the setting of a prostatic abscess. Chronic bacterial prostatitis is seen in patients with repeated urinary tract infections, most commonly E. coli. Patients will present with dysuria and pelvic or low back pain. Positive urinary cultures can be seen in between periods of symptomatic bacteriuria. Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) represents the most common type. Numerous medical therapies have been tried, but a definitive treatment still remains unknown. Patients in this subtype complain of LUTS and pelvic pain. Treatments to consider consist of anti-inflammatories, alpha-adrenergic blockers, and hormonal therapies. A recent study by Kong et al. evaluated the use of phosphodiesterase inhibitors, specifically mirodenafil, in combination with levofloxacin. The group receiving mirodenafil noted an improvement in LUTS; however, pain was unchanged [25]. CP/CPPS is further broken down into inflammatory and noninflammatory. Noninflammatory chronic pelvic pain syndrome is characterized by the absence of WBCs in prostatic fluid and no prostatic bacterial infection. Inflammatory CP/CPPS also called nonbacterial prostatitis is defined as WBCs in prostatic fluid, but no active prostate infection. The etiology may be related to reflux of urine into the prostate causing an irritant prostatitis. Asymptomatic inflammatory prostatitis is seen during the workup for other genitourinary pathologies such as following a prostate biopsy. A common finding is a large concentration of leukocytes in semen [26]. Granulomatous prostatitis, which is not included in the NIH classification, is seen following bacillus Calmette-Guérin (BCG) intravesical therapy for bladder cancer and tuberculosis.
TRUS
Transrectal ultrasound to diagnose prostatitis is generally not used since the diagnosis is clinical; however, TRUS should be considered if there is clinical suspicion for a prostatic abscess. A study involving 45 men with a clinical diagnosis of acute bacterial prostatitis who underwent TRUS on admission and 1 month following antibiotic therapy demonstrated a mean reduction in prostate volume from 40.5 mL (sd: 17.9) to 24.3 mL (sd: 10.5) [27]. Several signs on TRUS were described in a 1989 article by Doble and Carter that have a high correlation with a prostatitis diagnosis. These included high-density and midrange echoes, echolucent zones, capsular irregularity and thickening, ejaculatory duct echoes, and periurethral zone irregularity. Their histopathological significance was also noted. Corpora amylacea were seen as the high-density echoes. Midrange echoes represented inflammation and/or fibrosis. The echolucent zones indicated inflammation. The specificity of midrange and high-density echoes was 51.9 % and 40.7 %, respectively. The sensitivity of the echolucent zones, capsular irregularity and thickening, ejaculatory duct echoes, and periurethral zone irregularity ranged from 30.8 to 62.3 % [28].
A study comparing TRUS findings in the evaluation of men with chronic pelvic pain syndrome and chronic prostatitis revealed a statistically significant accumulation of prostatic calcifications and unilateral seminal vesicle gland alterations in men with chronic prostatitis. In patients with chronic pelvic pain syndrome, 22 % had prostatic calcifications and 11 % showed unilateral seminal vesicle gland alterations [29].
Color Doppler TRUS has been shown to be of value for monitoring acute bacterial prostatitis response to treatment. A study involving 25 patients treated for acute bacterial prostatitis had color Doppler and TRUS done at 1 week, 6 weeks, 3 months, and 6 months follow-up. The patients completed antibiotic therapy at 6-week follow-up ultrasound. 23 of the patients had blood flow to the entire prostate capsule at 1 week, and only two patients had blood flow to the entire capsule at 6 months. The patients in the same study were also noted to have an overall decrease in the amount and distribution of blood flow within the parenchyma based on a quantification system by the authors. They used the Doppler spot scale where they counted blood flow spots in the parenchyma and noted a decrease from mean 23.2 (sd: 11.1) spots at 1 week TRUS down to 7.9 (sd: 5.1) at 6 months [30].
MRI
The utilization of MRI in active surveillance for prostate cancer leads to more imaging of granulomatous prostatitis induced in patients previously treated with intravesical BCG for bladder cancer. Granulomatous prostatitis can mimic prostate cancer and may appear as a new hypointense lesion on T2W images with restricted diffusion. This was observed in a case report by Logan et al. about a patient undergoing multiparametric prostate MRI for active surveillance. These benign lesions may prompt earlier prostate biopsies [31]. Nagel and colleagues were able to show that diffusion-weighted MRI can show differences between prostatitis and prostate cancer in the peripheral zone and transition zone [32].
Prostatic Abscess
A prostatic abscess, though uncommon, should be suspected when a patient being treated for bacterial prostatitis fails to improve with antibiotic therapy. E. coli is the most common causative organism and is typically caused by the reflux of infected urine into the prostate gland. Additionally, hematogenous spread usually secondary to Staphylococcus aureus and direct inoculation following a prostatic procedure can lead to formation of a prostatic abscess. Patients may present with LUTS, perineal pain, fever, chills, low back pain, or even sepsis [33]. A fluctuation in areas of the prostate can be noted on digital rectal examination, but will not always be apparent. Immunosuppression and uncontrolled diabetes place a patient at an increased risk. Management is antibiotic therapy with abscess drainage. Surgical intervention includes transurethral unroofing and resection, perineal incision and drainage, and percutaneous drainage. Recurrence of a prostatic abscess can occur following initial treatment, so repeat imaging to confirm resolution of the abscess is recommended. This is commonly done with TRUS; however, CT and MRI are feasible options [33].
TRUS
Transrectal ultrasound is the diagnostic study of choice for a prostatic abscess. Hypoechogenic areas of varying sizes, which can be numerous, are a common finding. These areas are usually found in the transition zone and can distort the normal anatomy of the gland [33]. A small retrospective study involving nine patients by Oliveira et al. found all patients undergoing TRUS to have the above-described findings [34].
CT
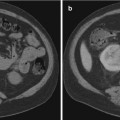
CT scan is another useful method to diagnose and follow a prostatic abscess (Fig. 5.2a, b). A small prospective study followed seven patients with suspected prostatitis. Five of the patients had a CT scan alone and showed a homogenous area of low attenuation on CT after giving IV contrast. Two of the patients demonstrated enhancing rims around the abscess. CT scan has demonstrated to be useful in diagnosing and to follow resolution of the abscess. Both CT and TRUS can reveal septations; however, CT allows evaluation of adjacent tissue structures [35].
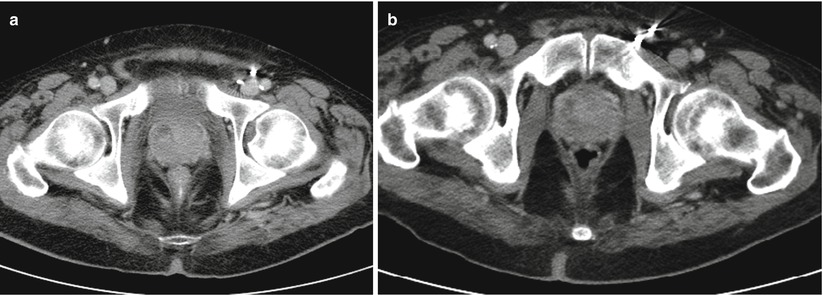

Fig. 5.2
(a) Contrast-enhanced CT scan, axial cut with a 1.3 × 1.2 cm hypoattenuating focus in the right anterior portion of the prostate with enhancement suggesting prostatic fluid collection concern for an abscess formation. (b) Contrast-enhanced CT scan, axial cut of the same patient several days later demonstrating a mild decrease in size of 0.7 × 0.5 cm
MRI
MRI will show a hypointense signal on T1 and hyperintense signal with peripheral enhancement on T2 [36] (Fig. 5.3a, b). Singh et al. evaluated the use of diffusion-weighted imaging (DWI) in two patients with a prostate abscess. DWI evaluates the diffusion of water molecules and calculates an apparent diffusion coefficient (ADC) between normal and pathological prostate. They demonstrated that the mean ADC levels in prostatic abscesses were low in comparison to normal peripheral zone prostate tissues and with malignant prostate tissue. The area of restricted diffusion represented an area of abnormality on T2 imaging [36].
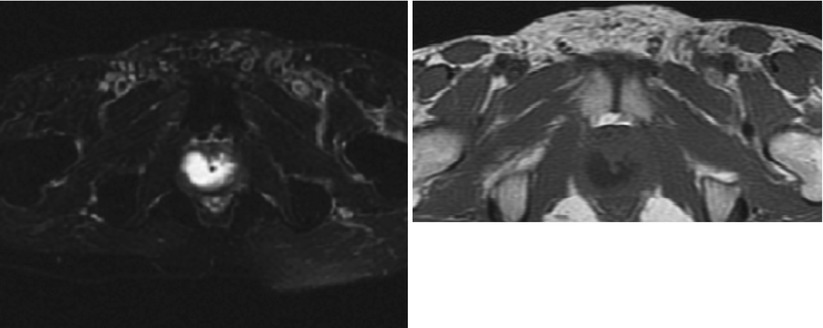

Fig. 5.3
(a) MR T2 axial cut of patient with suspected prostatic abscess and enlarged prostate. An intraprostatic periurethral high T2 signal suggesting fluid. (b) MR T1 axial cut of the same patient with suspected prostatic abscess and enlarged prostate. An intraprostatic periurethral abscess collection appearing as low signal on T1
Prostatic Cysts
A variety of cysts can exist in the prostate and can be asymptomatic or symptomatic depending on their origin and size. The overall prevalence of prostatic cysts is not known, but studies have shown medial cysts in up to 8 % of asymptomatic patients. Another small study found prostatic utricle (PU) cysts in 11 % of 150 infertile men, while no cysts were found in 30 fertile men. Prostatic cysts can be broken down into intraprostatic and periprostatic. Treatment is generally reserved for those cysts that may be associated with urinary obstruction, infertility, infection, or discomfort. The classification of prostatic cysts is based on their anatomic location which includes midline cysts, paramedian cysts, lateral cysts, and periprostatic cysts [37].
Galosi et al. proposed a new classification of prostatic cysts based on clinical and pathological features. They further described the appearance of the cystic lesions based upon imaging. Previous classifications have based cysts on clinical settings which included LUTS, oncology, infertility, and infectious disease [37]. They divided the cysts into six categories identified as type 1 through 6 in order: isolated medial cysts, ejaculatory duct cysts, parenchymal cysts, complicated cysts (hemorrhagic or infectious), cystic tumors, and parasitic cysts [37].
Midline cysts consist of PU cysts and Müllerian cysts. Müllerian cysts originate from remnants of the Müllerian duct, while PU cysts come from dilation of the prostatic utricle. These terms are sometimes used interchangeably; however, most literature classifies them as different entities. Midline cysts are located superior to the verumontanum in between the seminal vesicles. Müllerian cysts and PU cysts may be indistinguishable from one another on imaging, but there are differences on imaging to tell them apart. Müllerian duct cysts can originate lateral to midline given the anatomic location of the Müllerian ducts during embryology and anywhere along the Müllerian duct path. PU cysts should be precisely midline given the anatomic location the prostatic utricle. Aside from anatomic location, PU cysts communicate with the posterior urethra. This feature is not seen in Müllerian duct cysts. Müllerian duct cysts tend to be larger and occur later in life, while PU cysts occur in the first two decades of life. Lastly PU cysts have been associated with genitourinary abnormalities, while Müllerian duct cysts are isolated entities. Galosi also identified an enlarged PU in children, which is not a true cyst and is associated with an absent verumontanum and wide opening into the urethra. The enlarged PU is a tubular structure with squamous epithelium. A study involving 65 patients with Müllerian duct cysts evaluated the workup and management. Of the 65 patients, 27 underwent an invasive procedure which included transperineal or transrectal puncture in 9, endoscopic resection of the utricle meatus in 12, and large marsupialization in 6 patients. They noted improvement or cure in 82 % of the patients at roughly 4 years follow-up [38]. Halpern and Hirsch discuss a case report of a 35-year-old male with infertility found to have a Müllerian duct cyst. TRUS was used to guide transurethral laser incision of the cystic obstruction. Following incision, TRUS confirmed cystic decompression [39].
Ejaculatory duct cysts are paramedian cysts which can be congenital or acquired due to an ejaculatory duct obstruction. Evidence for an acquired ejaculatory duct cyst can be seen when there is a prostatic calculus or PU cysts. These cysts when aspirated contain spermatozoa or fructose [40].
Prostatic retention cysts are acquired and increase in frequency with advancing age in patients with BPH in the fifth and sixth decades of life. They result from the obstruction of prostate gland ductules resulting in upstream cystic dilation of the gland acini. On aspiration, spermatozoa are absent. BPH with cystic degeneration is the most common cystic lesion of the prostate and located in the transitional zone of the prostate gland [40]. They can be seen within the hyperplastic nodules and contain calculi and white or hemorrhagic fluid. The white fluid is corpora amylacea and hemorrhage is due to necrosis or infarction of prostatic nodules [40].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree