Chemoradiation for head and neck cancer is associated with a variety of early and late complications. Toxicities may affect the aero-digestive tract (mucositis, salivary gland injury), regional osseous and cartilaginous structures (osteoradionecrosis (ORN) and chondronecrosis), vasculature (progressive radiation vasculopathy and carotid blow out syndromes), and neural structures (optic neuritis, myelitis, and brain injury). These may be difficult to distinguish from tumor recurrence on imaging, and may necessitate the use of advanced MRI and molecular imaging techniques to reach the correct diagnosis. Secondary radiation-induced malignancies include thyroid cancer and a variety of sarcomas that may manifest several years after treatment. Checkpoint inhibitors can cause a variety of adverse immune events, including autoimmune hypophysitis and encephalitis.
Key points
- •
Complications from chemoradiation for head and neck malignancy may arise days to years after treatment.
- •
The radiologist’s task while interpreting surveillance imaging studies is not only to detect tumor recurrence but also identify complications that may have arisen in regional osseous structures, soft tissues, vasculature and neuraxis.
- •
The imaging appearance of some of these may mimic that of tumor recurrence.
- •
Clinical and imaging features of some complications such as optic neuritis and myelitis may be nonspecific and a history of prior radiation is necessary for correct diagnosis.
- •
Checkpoint inhibitors are a class of novel immunotherapeutic agents that are associated with a variety of unique toxicities with imaging manifestations that radiologists must learn.
Introduction
The treatment of cancers of the head and neck, by the virtue of their proximity to and tendency to arise from and involve anatomic structures vital for deglutition, speech, and respiration, frequently results in adverse effects compromising one or more of these functions. Aggressive management of these tumors by combining radiation and chemotherapy, the latter whether administered as induction treatment or concurrently, has led to superior disease control, albeit with a higher toxicity cost. Preservation of organ function and minimization of toxicity remain major goals in the use of chemoradiation in disease management. Although a wide range of adverse phenomena may occur during or after the course of chemoradiation in patients with head and neck cancer, this review will focus on the imaging manifestations of those that affect the brain and the osseous and soft tissue structures of the head and neck.
Clinical considerations
“Toxicity” may include any temporary or permanent change in normal tissues and/or related symptoms arising from cancer treatment and may be local or systemic. Adverse events arising from chemoradiation may be “early” or “late” in nature. Traditional chemotherapeutic agents used in head and neck cancer are not only inherently associated with a wide range of toxic effects but also may exacerbate the adverse effects of radiation. The emergence of immunotherapy with immune checkpoint inhibitors (ICI) has caused several widespread systemic immune-related adverse events that can cause significant morbidity and, rarely, mortality. Early, acute adverse events are usually temporary and are defined based on observations from conventional fractionated radiation as those encountered within 90 days of commencement of treatment. Most late events arise in the first 3 years of treatment with rare long-term sequela noted thereafter and may be permanent. The development of newer, more aggressive fractionation techniques and chemoradiation schedules has resulted in early toxicities manifesting beyond 90 days. Early toxic events primarily affect oropharyngeal and laryngeal function; mucositis is the most commonly encountered intensity limiting adverse effect. , Xerostomia due to salivary gland injury is also common and may be minimized using highly conformal radiation techniques designed to spare salivary gland tissue
Early adverse events from chemotherapy may include mucositis, exacerbation of radiation-induced dermatitis, hypersensitivity reactions, myositis, endocrinopathy, myelosuppression, neuropathy, and nephrotoxicity. Late complications after radiation include dental injury (exacerbated by impaired salivary function), osteoradionecrosis (ORN), radiation vasculopathy, radiation necrosis of brain tissue, optic neuritis, myelitis, thyroid dysfunction, and rarely second malignancies.
Mucositis
Mucositis is a near universal, painful complication of cancer treatment. It is a biologically complex process believed to evolve through 5 cellular phases. Its pathologic hallmark is ulceration arising from the loss of the basal epithelial/stem cell layer. Clinically evident mucositis is comprised of a confluence of such ulcers, covered by a pseudomembrane of inflammatory cells, cellular debris, fibrin, and exudate. , Mucositis becomes evident in the first few weeks after radiation but can persist for weeks to months thereafter. Failure of healing may lead to permanent ulceration and mucosal necrosis. On computed tomography (CT) studies obtained shortly after treatment ( Fig. 1 ), affected mucosa demonstrates a low density, edematous “boggy” appearance. Studies often demonstrate other changes in the superficial soft tissues of the neck including the reticulation of the subcutaneous and deep fat, often accompanied by the thickening of the skin, fascia and platysma, and retropharyngeal edema and effusion. Mucositis must be distinguished from frank mucosal and soft tissue necrosis ( Fig. 2 ) that occurs in the first few years after radiotherapy. On CT and MR imaging, this presents as an irregular debris containing ulcer. Although lack of enhancement is a reassuring finding, this may be associated with enhancement that is indistinguishable from tumor recurrence. In the latter scenario, close observation and/or biopsy may be required for definitive diagnosis. ,


Salivary gland injury
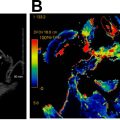
Xerostomia is a common, long term, dose-dependent complication of radiotherapy. Severe xerostomia may be avoided by minimizing dose and/or using highly conformal radiotherapy techniques to minimize parotid and submandibular gland exposure. The salivary glands exhibit early and late changes after chemoradiation with dramatic reduction in salivary flow rates noted in the first 2 weeks after treatment and gradual glandular atrophy developing due to the loss of acinar cells approximately 6 weeks thereafter. Parotid glandular volume typically decreases soon after radiotherapy, with a degree of recovery occurring thereafter when parotid sparing radiation techniques are used. On CT studies obtained soon after radiation, increased gland density may be apparent with correspondingly increased T2 signal on MRI due to edema ( Fig. 3 ). Increased vascular permeability and acinar loss in the parotid glands may underlie changes observed on dynamic contrast-enhanced perfusion MRI and intravoxel incoherent motion MR imaging parameters as soon as 2 weeks after radiotherapy. ,

Thyroid gland injury
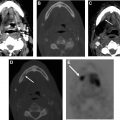
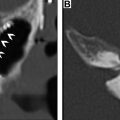
Hypothyroidism, necessitating routine life-long screening with serum thyroid-stimulation hormone, develops in about one-third of patients undergoing external beam radiation for head and neck cancers, usually within the first 2 years after treatment and is more common in patients who also undergo surgery. Checkpoint inhibitors may also cause autoimmune thyroid dysfunction. Morphologic changes after external beam radiation, including gland shrinkage (and rarely, enlargement) and development of cystic and solid masses, are well-documented on cross-sectional and ultrasound studies. , In a CT study, 85% of patients demonstrated a decrease in thyroid gland width after radiation for laryngeal cancer, with change in gland size being no different in patients with hypothyroidism and those without. Pre-existing thyroglossal duct cysts may also enlarge after radiation ( Fig. 4 ), usually evident on first posttreatment studies due to inflammation and must not be mistaken for metastatic nodal disease. The evidence for a causal relationship between radiation exposure and the development of thyroid cancer, especially of the papillary type, is incontrovertible. Although screening of all individuals exposed to radiation by yearly physical examination is recommended, the use of ultrasound is controversial. The benefits of early tumor detection must be balanced against the risks of unnecessary and expensive treatments and their attendant complications for tumors that may remain indolent and never become clinically apparent. Surveillance ultrasound is offered widely to survivors of childhood cancer that have received radiation to the head and neck, given the greater likelihood of developing clinically relevant thyroid malignancies.

Osteoradionecrosis
ORN, although most commonly encountered in the mandible after radiation for oral cavity cancers, may affect any bony structure exposed to radiation in the head and neck. ORN may occur a few months to several years after radiation. The clinical presentation is variable and ranges from incidentally seen asymptomatic areas of exposed bone that may heal with conservative management to widespread bone loss necessitating extensive surgical reconstruction. ORN may present with pain, halitosis, dysgeusia, dysphagia, speech difficulty and with erythema, soft tissue swelling and abscess, and fistula formation when there is co-existent osteomyelitis.
Its pathogenesis involves the development of hypoxia from radiation-induced vascular obliteration causing tissue breakdown and a clinically evident nonhealing wound, frequently containing exposed bone, with microorganisms playing only a contaminant role. The risk of developing ORN is related to tumor T stage, proximity of tumor to bone, radiation dose and, perhaps, technique, trauma, dentition, nutritional status, alcohol abuse, smoking, underlying cardiovascular disease, and bisphosphonate use.
Although studies indicate a decreasing prevalence of ORN attributed to newer radiation techniques, improved awareness and preventive care, others contend that newer radiation modalities have not had a measurable impact on its occurrence; a meta-analysis revealed similar prevalence rates of ORN between conventional radiotherapy (7.4%), intensity-modulated radiation therapy (IMRT) (5.1%), chemoradiation (6.8%), and brachytherapy (5.3%).
The CT findings ( Fig. 5 ) of isolated ORN include bone erosion (frequently of the lingual mandibular cortex adjacent to the molar teeth being an early sign), loss of trabeculations, intraosseous gas, fragmentation, sclerosis, and fracture. On MRI, marrow edema (evident as low T1 and high T2 signal) is typical. The absence of associated soft tissue abnormality may enable differentiation of ORN from tumor recurrence, but is not always straightforward. , Coexistent osteomyelitis may result in adjacent soft tissue edema, periostitis, and formation of abscesses and sinus tracts. Imaging signs demonstrating signs of osteomyelitis in irradiated bone likely indicate underlying ORN. Likewise, the presence of a solid or necrotic mass adjacent to affected bone may indicate tumor recurrence and must prompt biopsy. PET imaging is of limited value in differentiating ORN from recurrence with significant overlap in standardized uptake value (SUV) values being observed between the 2. ORN of the cervical spine, a rare complication of radiation for nasopharyngeal, hypopharyngeal, and skull base cancers, may be difficult to differentiate from metastatic disease. However, contiguous, symmetric, upper cervical vertebral involvement, vertebral body collapse, and posterior pharyngeal wall soft tissue ulceration without paraspinal or epidural soft tissue components may help differentiate them.

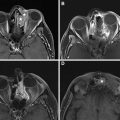
Radiation for nasopharyngeal, parotid, and external auditory canal malignancy rarely may result in temporal bone and skull base ORN ( Fig. 6 ), with patients presenting with otalgia, hearing loss, and purulent otorrhea. Imaging findings are similar to those encountered at other sites and include erosions of the bony external auditory canal in the early stages, mottled bone loss, mastoid and middle ear effusions, intraosseous gas, and destruction of the temporomandibular joint. In rare cases, soft tissue and intracranial abscesses and meningitis may occur due to superimposed infection.

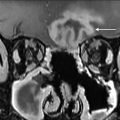
Laryngeal chondroradionecrosis (LCRN) ( Fig. 7 ) presents with throat pain, hoarseness, dyspnea, and dysphagia and occurring soon after or several years after radiation. It is more likely to occur after the treatment of tumors that involve cartilage at presentation. The Chandler grading system classifies LCRN into 4 grades of progressively increasing severity with patients with grade IV disease (severe pain and odynophagia, weight loss and laryngeal obstruction causing severe respiratory distress) requiring laryngectomy. LCRN occurs due to radiation-induced end arteriolar obstruction in the perichondrium that nourishes cartilage. Findings on CT include cartilage fragmentation, collapse, and sloughing and gas bubbles within and around cartilage. PET imaging can show hypermetabolism, making distinction from tumor recurrence difficult.

Mild/early-onset ORN is managed conservatively using a combination of debridement and antibiotics. Other approaches include the administration of hyperbaric oxygen after definitely ruling out tumor recurrence, although its efficacy remains controversial, and combination treatment pentoxifylline, vitamin E, and clodronate. , More severe ORN with large areas of bone exposure, discharging fistulae, or associated with fracture may require radical sequestrectomy or segmental mandibulectomy and free flap reconstruction.
Ophthalmological complications
Radiation-induced optic neuropathy (RION) occurs due to ischemic necrosis of the anterior visual pathways. Vision loss usually occurs approximately 18 months (range 7–48 months) after treatment, is often profound, acute in onset and may be mono- or binocular, simultaneous, or sequential and is frequently irreversible. Although the risk of RION increases with radiation dose, it may occur in patients at doses below “safe” radiation thresholds (<55 Gy; <8–10 Gy for stereotactic radiosurgery). Increased T2 signal, subtle thickening, and enhancement of the prechiasmatic segment of the optic nerve(s) are typical findings on MRI ( Fig. 8 ). It is important to note that these findings may be subtle, precede the development of visual loss and may persist for several months after treatment. Management includes corticosteroids and hyperbaric oxygen, although no definitively effective therapeutic measures exist. Ocular complications may also include keratoconjunctivitis, xerophthalmia from lacrimal gland injury, nasolacrimal duct damage, injury to the iris and choroid, cataracts, and retinopathy.

Ototoxicity
Radiation can affect all components of the auditory pathway. The median dose for the occurrence of ototoxicity after radiation for head and neck cancer is 60 to 66 Gy and above, although the threshold may be even lower (32–50 Gy). , Concurrent cisplatin administration significantly increases the risk of sensorineural hearing loss. The threshold cochlear dose for hearing loss with concurrent cisplatin-based chemotherapy may be as low as 10 Gy. Rarely, enhancement of the labyrinthine structures may be evident after radiation on contrast-enhanced MRI ( Fig. 9 ).

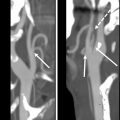
Myelitis
Radiation myelopathy may occur a few months to several years after the treatment of head and neck cancer with radiation. The devastating nature of this entity compels the oncologist to prioritize excluding the spinal cord over tumor coverage during radiation planning and limiting total dose to the cord to less than 45 Gy in daily 1.8 to 2.0 Gy fractions. The clinical and imaging manifestations of radiation myelopathy may be nonspecific, prompting consideration of other inflammatory, demyelinating, and autoimmune conditions unless a history of prior radiation is recognized. MRI abnormalities include long-segment cord T2 signal abnormality, cord expansion, and variable hemorrhage and enhancement with gadolinium contrast ( Fig. 10 ). Khan and colleagues suggest that predilection for this entity to affect the central gray matter to a greater degree than the white matter tracts in its periphery perhaps implies ischemia and vasculitis as its underlying cause rather than demyelination. The presence of T1 hyperintense signal in the cervical vertebral bodies due to fatty marrow replacement in the radiation field is a useful clue to the diagnosis. Cord atrophy is frequently seen in survivors with a minority of patients demonstrating complete resolution of MRI changes. ,