Gynecologic malignancies are common among cancers diagnosed during pregnancy, especially those of cervical and ovarian origin. Imaging is an important part of the diagnosis, staging, and follow-up of pregnancy-associated gynecologic tumors, with sonography and magnetic resonance (MR) imaging being the most suitable modalities. MR imaging is particularly useful in cervical cancer for the evaluation of tumor size, nodal, and extrapelvic disease. Ovarian tumor is initially diagnosed with sonography; MR imaging should be performed in cases of indeterminate ultrasonography findings and for staging. Pregnancy-related changes may be responsible for erroneous diagnosis; radiologists should be aware of such pitfalls to avoid misinterpretation.
Key points
- •
Cervical cancer is the most common gynecologic cancer during pregnancy. Magnetic resonance (MR) imaging is the preferred imaging modality for cervical cancer staging in the gravid population.
- •
Most ovarian tumors in pregnant patients are benign but they occasionally undergo pregnancy-related morphologic changes mimicking malignancy.
- •
Sonography is the initial imaging modality of choice for the evaluation of ovarian masses in the pregnant population. MR imaging is valuable when sonography is inconclusive, when the mass is too large, and for staging potentially malignant lesions.
- •
Uterine, vaginal, or vulvar cancers are extremely rare in the gravid population; imaging is used for determining the extent of the disease.
Introduction
Pregnancy-associated cancer includes any cancer diagnosed from the day of conception to 12 months after delivery to account for possible delays in diagnosis during the course of pregnancy and because postpartum cancer is considered a continuum of cancer in pregnancy ( Box 1 ). ,
Any cancer diagnosed from the day of conception to 12 months after delivery.
Cancer in pregnancy is uncommon, with a reported incidence ranging from 0.02% to 0.1%. Several epidemiologic studies suggest an increase in pregnancy-associated cancers in recent years. , Advanced maternal age is considered a strong predisposing factor, because the incidence of cancer increases with age ; however, the increased incidence of pregnancy-associated malignancies cannot be explained only by the delay in childbearing. ,
Among cancers diagnosed during pregnancy, gynecologic malignancies are common, especially those of cervical and ovarian origin ( Box 2 ). Recently, in an international cohort of 1170 women with pregnancy-associated cancer, gynecologic tumors accounted for 20% of the cases (cervical cancer, 13%; ovarian cancer, 7%); breast cancer and hematological malignancies were diagnosed in 39% and 16% of the study patients, respectively. A similar incidence of cervical (15%) and ovarian cancer (6%) was also reported in a recent study performed in the Swedish population.
Breast cancer
Hematological malignancies
Cervical cancer
Ovarian cancer
Note that pregnancy status does not seem to adversely affect maternal survival compared with nonpregnant patients treated for cancer and, furthermore, outcomes concerning fetal safety are encouraging. Early diagnosis of cancer is extremely important for successful treatment, regardless of gestational age, because advanced disease at the time of diagnosis is strongly associated with poorer prognosis. However, there is often a delay in the diagnosis of pregnancy-associated cancer for several reasons. Common symptoms of malignancy, such as nausea, vomiting, pain, anemia, fatigue, or even vaginal spotting or bleeding, overlap with symptoms of pregnancy. Physical examination of breasts and abdomen is difficult because of hormonal changes and the enlarged gravid uterus. Commonly used tumor markers (eg, cancer antigen [CA] 15-3 for breast cancer, CA 125 for epithelial ovarian cancer, or alpha fetoprotein for germ cell tumors) are unreliable because they may be normally increased in pregnancy. Moreover, physicians hesitate to use imaging studies other than sonography, particularly in the first trimester of pregnancy, to avoid any harmful effect on the fetus. For all these reasons and because cancer during pregnancy is a rare event, it may not be considered in the differential diagnosis of early symptoms.
Imaging techniques applied in pregnancy
Imaging of pregnant patients with cancer is challenging because conflict between maternal benefit and fetal risk needs to be addressed. Before selecting the appropriate imaging modality for the diagnostic and staging work-up of pregnant patients with cancer, several issues need to be considered: fetal health (closely related to pregnancy trimester), maternal survival (closely related to tumor staging and possibility of metastases), and desire to continue the pregnancy.
It is generally suggested that imaging of the pregnant population is to be restricted to those methods that do not endanger fetal health and particularly, during the first trimester of pregnancy, only necessary radiological investigations are justified. At present, sonography and magnetic resonance (MR) imaging are acceptable imaging modalities for assessing pregnant patients because they lack ionizing radiation.
Sonography is the modality of choice for the initial abdominal evaluation of gravid patients because it is widely available, low cost, and lacks adverse effects to mother and fetus. However, prolonged use of color Doppler imaging during the first trimester is better avoided, because there is a theoretic risk for tissue heating caused by acoustic energy deposition; appropriate settings for obstetric imaging minimize such risk. However, as the pregnancy progresses, the enlarged gravid uterus obscures the pelvic organs and retroperitoneal space, rendering sonography less suitable for examination of the abdomen in patients with gynecologic malignancies.
Because of larger imaging fields of view, better reproducibility, and excellent soft tissue contrast, MR imaging is considered the imaging modality of choice for diagnosis and staging of gynecologic cancer during pregnancy. According to the American College of Radiology (ACR) guidelines, pregnant patients can be safely imaged with field strengths up to 3 T using a normal-level specific absorption rate (SAR) mode (<2 W/kg) with no adverse effects on organogenesis or fetal hearing documented. , Contrast-enhanced MR imaging may be used with caution and only when there is a significant benefit to the mother that outweighs the risk of fetal exposure to paramagnetic media, because gadolinium-based contrast agents cross the placenta-blood barrier. Recently, whole-body diffusion-weighted imaging (DWI) has been proposed for oncologic staging of pregnant patients because it shows promising results and eliminates the need for intravenous contrast ; however, large prospective studies are needed to support its clinical implementation ( Box 3 ).
No special consideration for any trimester with field strengths up to 3 T
No known adverse effects on organogenesis or fetal auditory function
Gadolinium-based contrast agent may be used with caution
Imaging with DWI shows early promising results
The use of ionizing radiation is best avoided during pregnancy, especially in the first 2 trimesters because of potential risk of teratogenesis; although the absorbed doses for diagnostic imaging even for abdominal computed tomography (CT) are usually less than 50 mGy and considered safe for the fetus, cumulative radiation and stochastic effects should also be taken into consideration. CT in gravid patients should be performed only when the information required cannot be obtained with sonography or MR imaging and only after careful risk-benefit assessment, always keeping the radiation dose to the fetus as low as reasonably achievable (ALARA principle). In all cases, appropriate consultation for potential fetal risks and written informed consent from the patient is strongly advised.
18F-fluorodeoxyglucose (FDG) PET/CT in pregnant patients is discouraged because of fetal exposure to radiation and potential toxicity of the radiopharmaceutical, even though reported total absorbed fetal doses are less than the threshold of 50 mGy. However, if it is medically indicated, PET/CT may be performed after careful risk-benefit assessment; PET/MR imaging can provide detailed imaging without the CT-related radiation, but its availability is still limited.
More details regarding safety concerns of imaging pregnant women, and women of reproductive age in general, are provided in Michael A. Ohliger and Hailey H. Choi’s article, “ Imaging Safety and Technical Considerations in the Reproductive Age Female ,” elsewhere in this issue.
Imaging protocols tailored for cancer in pregnancy
Most investigators agree that it is difficult to advocate a single comprehensive protocol for maternal imaging in the setting of pregnancy, because several parameters must be considered, including clinical indications, gestational age, and technical issues. Imaging protocols should focus on answering a specific clinical question, taking into account fetal safety and maternal comfort and balancing short imaging times with acceptable image quality.
Magnetic Resonance Imaging: Preparation
MR imaging of the abdomen and pelvis in pregnant patients is technically challenging ( Box 4 ). It is not only maternal breathing and bowel peristalsis but also fetal motion that may degrade image quality. The effects of peristalsis and fetal motion may be reduced by asking the patient to fast for approximately 4 to 6 hours before the MR imaging study ; antiperistaltic agents (eg, 20 mg of hyoscine butyl bromide, intramuscularly) may be administered before the examination, because there is no contraindication for their use during pregnancy. The impact of fetal movement can be further minimized with the use of ultrafast sequences (single shot fast spin echo). The urinary bladder should be moderately distended to allow adequate evaluation of its wall without causing maternal discomfort. Pregnant patients are typically imaged in the supine position. However, especially in the third trimester of the pregnancy, it may be difficult for the patient to suspend respiration and remain in the supine position for a long time. As the gravid uterus enlarges, it may cause pressure on abdominal organs and vessels, including the inferior vena cava. In such cases, imaging may be performed in the left lateral decubitus position to avoid impairing venous return from the pelvis and lower extremities, which may result in dizziness or even syncope. Use of a surface phased-array coil is recommended because of superior signal/noise ratio and better contrast resolution. However, in larger patients with advanced pregnancy, imaging may be performed with the body coil.
Ask the patient to fast for 5 to 6 hours before imaging
May use antiperistaltic agents
Bladder should be moderately full (tip: ask the patient to void and then give her 1–2 cups of water, approximately 20–30 minutes before entering the scan room)
Use surface coil if possible
Apply ultrafast sequences as required
In late pregnancy, imaging may be performed in the left lateral decubitus position
Magnetic Resonance Imaging: Sequences
There is insufficient data in the literature regarding MR imaging protocols in pregnant patients with gynecologic tumors. Therefore, oncologic protocols used in nonpregnant patients with suitable adjustments are mostly used. Overall scan time should not exceed 30 to 40 minutes because of concerns for tissue heating and maternal discomfort.
An example of an MR imaging protocol used to evaluate gynecologic malignancies in nonpregnant patients according to the European Society of Urogenital Radiology guidelines, , with modifications based on the needs of each pregnant patient, is presented in Table 1 .
| Sequence | Plane | Value |
|---|---|---|
| T2-W TSE (mandatory) |
| Allows an overall view of the pelvic and abdominal organs and minimizes fetal motion artifacts |
| High-resolution T2-W TSE, with limited field of view in the pelvis (mandatory) |
| Achieves optimal contrast difference between tumor and normal tissue. Fetal motion artifacts only rarely significantly compromise image interpretation |
| T1-W ±fat suppression (mandatory) TSE or Dixon |
| Provides information on pelvic anatomy, hemorrhagic lesions, lymph nodes, and bone marrow. Fat suppression allows the diagnosis of hemorrhage vs fat and is particularly valuable in cases of ovarian tumors A Dixon technique reduces the number of sequences, but it is prone to artifacts from fetal movement |
| T2-W with fat suppression (optional) |
| Detects fluid collections and bone marrow changes |
| DWI (0, 800, 1400 s/mm 2 ) (mandatory) |
| Discriminates cancerous from normal tissue and detects lymph nodes |
| T1-W (2D or 3D GRE) DCE; not as routine |
| If contrast-enhanced imaging is required in case of ovarian tumors, use a macrocyclic gadolinium chelate at the lowest dose (0.1 mmol/kg) Use contrast media only if the information gained is critical for decision making |
Computed Tomography
CT scan of the abdomen and the pelvis is justified in pregnant women with cancer if there is no other diagnostic alternative. Radiation dose-reduction techniques should be applied, including decreasing voltage (kilovolts) and current (milliamps), increasing pitch, widening beam collimation, limiting scanned areas, and performing a single-phase study. Automated settings may reduce dose by 40% to 50% while maintaining acceptable imaging quality.
CT examinations with the fetus outside the field of view can be safely performed during any trimester of pregnancy, because fetal absorbed doses do not exceed 50 mGy; abdominal shielding may be used for protection from outside scatter radiation.
No teratogenic or mutagenic effects have been documented regarding the use of iodinated contrast media either in vivo or in vitro; however, their administration is recommended only under special circumstances and the exposed neonate should be checked for hypothyroidism soon after birth.
Cervical cancer in pregnancy
Epidemiology
Cervical carcinoma is the most common gynecologic malignancy in the gravid population, with an estimated incidence ranging from 0.05% to 0.1%. Cervical neoplasia (including intraepithelial lesions, in situ and invasive carcinomas) is expected to complicate 1.5 to 12 of 100,000 pregnancies, with invasive cancer affecting approximately 0.8 to 1.5 of 10,000 pregnancies. , About half of these cases are diagnosed during pregnancy, and the rest in the early postpartum period (within 12 months after delivery). With the increased use of screening, most (up to 70%) cervical cancers of pregnancy are diagnosed at an early stage with small-volume disease (stage 1B) , ; gravid patients with cervical cancer are 3 times more likely to have early disease (stage I) at diagnosis than nonpregnant patients. ,
Relative to histology, there is no difference between cancer types of pregnant versus nonpregnant women, with most cases (90%) being of squamous cell origin, followed by adenocarcinomas.
Diagnosis and Staging
The diagnostic approach for pregnancy-associated cervical cancer is similar to that in nonpregnant women. Recommendations include routine performance of Pap smear at the first prenatal visit; in women with high-grade intraepithelial lesions, colposcopy and biopsies should follow, because endocervical curettage is contraindicated. Colposcopy can be performed regardless of the pregnancy trimester; assessing the lesion’s extent may be challenging because of pregnancy-related edema and increased vascularity of the vagina and cervix.
The presence of symptoms is related to the clinical stage and tumor size. Common symptoms of invasive cervical cancer include vaginal bleeding or discharge, and abdominal/pelvic pain, which may be mistaken for threatened miscarriage or prepartum hemorrhage.
In cases of biopsy-confirmed invasive cervical cancer, staging work-up is indicated. The International Federation of Gynecology and Obstetrics (FIGO) classification system for staging cervical cancer may be applied to the pregnant population. Clinical examination is the first step of staging patients with cervical cancer. However, in the pregnant population, bimanual physical examination may be technically difficult or painful and, therefore, less sensitive for determining the size and lateral extension of a cervical mass; also, it provides no information regarding nodal status, the most adverse prognostic factor for this disease (stage IIIC disease according to the revised FIGO staging system).
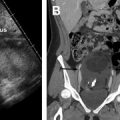
New FIGO guidelines (2019) have incorporated imaging data into the staging system for cervical cancer; the use of imaging modalities, including sonography, CT, MR imaging, and PET (according to available resources), is permitted to provide information on tumor size, nodal status, or systemic tumor spread, and may influence the choice of treatment. MR imaging is the preferred imaging technique for staging cervical cancer in gravid patients because it is safe for fetuses, reproducible, and non–operator dependent with excellent soft tissue contrast resolution and larger imaging field of view compared with sonography. It can accurately assess tumor size (all 3 dimensions); vaginal, parametrial, or adjacent organ invasion; hydronephrosis; and lymph node involvement. , Reported MR imaging accuracy values in the nonpregnant population for determining early (<IIB) versus advanced (≥IIB) disease are high, ranging from 75% to 96%. Clinical staging of early cervical cancer significantly improves when MR imaging information is added to the clinical data, enabling better selection of candidates for surgery. , Although, the clinical impact of pelvic MR imaging in cervical cancer of pregnancy has not been assessed in large cohorts, several investigators advocate its use for staging purposes in pregnant patients with pathologically confirmed cervical cancer and macroscopically visible disease (>IB1) or clinical signs suspicious for metastases. Alternatively, if MR imaging is not feasible, because it is contraindicated or the patient has severe claustrophobia, CT or even PET-CT may be used to evaluate tumor local extent and nodal enlargement, after careful patient consultation.
Imaging Findings, Differences from Nonpregnant Patients, and Potential Pitfalls
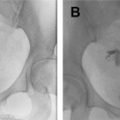
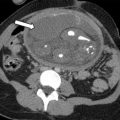
MR imaging features of cervical cancer during pregnancy are similar to those observed in nonpregnant patients, although there are some differences that should be taken into account during interpretation. During pregnancy, the normal cervical stroma may be hyperintense because of pregnancy-related edema and, therefore, tumor may be difficult to detect on conventional T2-weighted images. In such cases, DWI is valuable because typically only cancerous tissue shows restricted diffusivity ( Fig. 1 ); because postbiopsy hemorrhage may be the cause of false-positive results on DWI, time interval between cone biopsy and MR imaging examination should be longer than 1 week.

Small (<1 cm) cervical tumors may be easily overlooked on non–contrast-enhanced MR imaging and may appear only on early arterial dynamic contrast-enhanced MR imaging as small enhancing foci. Although contrast enhancement may increase small lesion conspicuity, it is usually avoided in pregnant women because of fetal safety considerations; detection of cervical lesions less than 1 cm does not affect treatment decisions or maternal prognosis.
When uterine-sparing surgery is planned, estimation of the distance between the tumor and the internal cervical os (ICO) is of utmost importance ; a tumor to ICO distance more than 1 cm is usually required for successful outcomes. Tumor extension to the ICO is a poor prognosticator for patient survival because it is associated with an increased incidence of nodal metastases. MR imaging is a noninvasive modality with high sensitivity (86%–91%) and specificity (94%–96%) values for evaluating tumor extension to the corpus uteri. However, in pregnant patients, ICO evaluation may be difficult because the gravid uterus distorts normal cervical anatomy and pregnancy-related edema may alter normal ICO T2 signal intensity; in such cases information derived from DWI may be helpful.
Metastatic lymph nodes are strongly associated with poor clinical outcome in patients with cervical cancer. Surgical lymphadenectomy is considered the gold standard procedure for the diagnosis of nodal metastases; several investigators report no adverse fetal outcomes when the procedure is performed before 20 weeks of gestation. Recently, in 2019, imaging criteria for nodal assessment have been introduced in the new FIGO recommendations for staging cervical cancer. If imaging findings indicate pelvic nodal metastases, stage allocation is IIIC1r; with pathologic confirmation (via laparoscopic resection) it becomes IIIC1p. Accordingly, imaging diagnosis of para-aortic nodal involvement is classified as stage IIIC2r and pathologic confirmation as stage IIIC2p disease. In all cases, the type of imaging modality or pathology technique used should be documented.
In pregnant women, care should be taken not to mistake dilated pelvic veins for enlarged pelvic lymph nodes in the axial plane; use of other planes (eg, coronal) is helpful to avoid misdiagnosis.
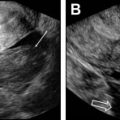
Another potential pitfall in pregnant women is the prolonged use of vaginal progesterone gel to avoid premature birth. This prolonged use may result in the formation of a solid mass within the vagina, mimicking a cervical tumor; knowledge of the patient’s history may raise suspicion of such a condition and the gel foam mass may be removed with colposcopy ( Fig. 2 ).

Treatment Options and Prognosis
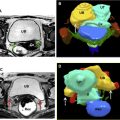
Management of invasive cervical cancer during pregnancy depends on the following criteria: tumor local extent (staging), nodal involvement, gestational age, and tumor histology ( Fig. 3 ). Uterine-sparing surgery and pregnancy preservation may be considered under certain circumstances. Current treatment options for cervical cancer during pregnancy are summarized in Box 5 . , Although there are no definitive conclusions about how overall prognosis differs between pregnant and nonpregnant patients with cervical cancer, it seems both groups have a similar survival profile. Recently, Cordeiro and colleagues reported that the 30-year survival of pregnant women diagnosed with cervical cancer is similar to age-matched and disease-matched controls, concluding that pregnancy does not adversely affect survival. Therefore, most investigators agree that pregnancy in women with cervical cancer can be preserved in most cases.