Infertility, or subfertility, is the inability to achieve a clinical pregnancy after a 1-year period of regular unprotected sexual intercourse in women younger than 35 and after 6 months in women older than 35. Although initial assessment involves a multitude of factors, including a detailed medical history, physical examination, semen analysis, and hormonal evaluation, diagnostic imaging of the female partner often plays an important role in establishing the etiology for infertility. This article provides an overview of the multimodality imaging assessment of female infertility and details the developmental and acquired pelvic abnormalities in which diagnostic imaging aids in evaluation.
Key points
- •
Infertility can be primary or secondary and affects up to 12% of couples of reproductive age worldwide.
- •
Causes of female infertility include congenital etiologies, such as Müllerian duct anomalies (MDAs), and acquired etiologies, which range from ovulatory dysfunction to cervical factors and uterine and tubal abnormalities.
- •
The imaging investigation of female infertility consists of a multimodality approach, and includes a combination of primarily ultrasound and hysterosalpingography (HSG), with MR imaging for problem-solving situations specifically if MDAs or deep infiltrative endometriosis are suspected.
- •
HSG remains the gold-standard imaging modality to assess tubal patency.
Introduction
Infertility, or subfertility, is defined as the inability to achieve a clinical pregnancy after a 1-year period of regular unprotected sexual intercourse in women younger than 35 and after 6 months in women older than 35. Prospective cohort studies have estimated the prevalence of infertility in the United States ranges from 12% to 18%. When couples undergo evaluation for infertility, the etiology is found to be 40% women and 40% men. It is a unique and often multifactorial medical condition that involves a couple, rather than a single individual, requiring a comprehensive approach for diagnosis and treatment. Causes of female infertility include congenital etiologies such as Müllerian duct anomalies (MDAs) and acquired ones, which range from ovulatory dysfunction to cervical factors and uterine and tubal abnormalities. Although initial assessment involves a multitude of factors, including a detailed medical history, physical examination, semen analysis, and hormonal evaluation, diagnostic imaging of the female partner often plays an important role in establishing the etiology for infertility. This typically includes a combination of various modalities, such as ultrasound, MR imaging, and primarily hysterosalpingography (HSG), depending on the suspected etiology. In addition to these traditional imaging modalities, we review some newer imaging techniques, such as hysterosalpingo-contrast sonography (HyCoSy) and virtual computed tomography (CT) and MR hysterosalpingography.
Anatomy
The female reproductive organs consist of the cervix, uterus, fallopian tubes, and paired ovaries. During the initial 6 weeks of gestation, both male and female fetuses possess paired Müllerian (paramesonephric) and Wolffian (mesonephric) ducts. In male fetuses, the presence of Müllerian-inhibiting factor, associated with the presence of a Y chromosome, results in the regression of the Müllerian ducts. In female fetuses, the absence of Müllerian-inhibiting factor leads to concomitant regression of the Wolffian ducts and growth of paired Müllerian ducts. Embryologically, the uterus, cervix, fallopian tubes, and upper two-thirds of the vagina are formed by fusion of paired Müllerian ducts. Failure or disruption of development of the ducts, such as absence of fusion or nonresorption or partial resorption of the midline septum following fusion will result in a complex spectrum of congenital abnormalities termed Müllerian duct anomalies (MDAs), some of which may be associated with infertility. The fallopian tubes measure 10 to 12 cm and are found running along the superior aspect of the broad ligament. Each tube is composed of 4 segments: interstitial, which is the shortest and narrowest and contained within the muscular portion of the uterus; isthmic, closest to the uterine wall; and ampullary and infundibular, which is the distal funnel-shaped fimbriated portion, opening into the peritoneal cavity. The fallopian tubes are not visualized unless they are dilated or surrounded by peritoneal fluid. The paired ovaries are ellipsoid, located in the ovarian fossa along the lateral pelvic wall. They are attached to the posterior aspect of the broad ligament by the mesovarium.
Imaging modalities
The imaging investigation of female infertility consists of a multimodality approach, and includes a combination of primarily ultrasound, HSG, and MR imaging for problem-solving situations or in cases of suspected MDAs. Each of these studies will focus on and help investigate different aspects of the infertility workup process ( Table 1 ).
| Imaging Modalities | Advantages | Disadvantages |
|---|---|---|
| Ultrasound |
|
|
| HSG |
|
|
| MR imaging |
|
|
| CT |
|
|
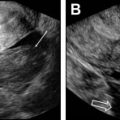
Pelvic ultrasound, performed both transabdominally and transvaginally (TV), is a first screening tool to insure the presence and integrity of the female reproductive organs. It has the advantage of being readily available, fairly inexpensive, relatively easy to perform, particularly in the hands of experienced operators, and lacks ionizing radiation. Ultrasound is excellent at diagnosing fibroids, endometrial polyps, adenomyosis/endometriosis, and imaging the ovaries to evaluate for polycystic ovaries, follicle development, and count antral follicles. Three-dimensional (3D) ultrasound is now used more routinely to assess the outside contour of the uterus in cases of suspected MDAs and has a reported 90% to 95% diagnostic accuracy. It also can accurately depict intracavitary lesions, such as polyps, submucosal fibroids, septum, and synechiae. Saline infusion hysterosonography (SIS), which involves the instillation of saline or gel through the endocervical canal using a catheter, allows delineation of the endometrium and its surface. This procedure can be performed using conventional 2-dimensional (2D) or 3D ultrasound. The latter consists of acquiring volumetric data that can be analyzed at a later time following the examination. This method allows for a shorter procedure time and less patient discomfort.
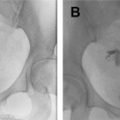
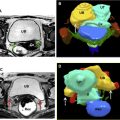
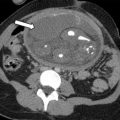
Dijkhuizen and colleagues found that SIS had a higher diagnostic accuracy in the detection of intracavitary uterine abnormalities when comparing it with TV scanning, with SIS having a sensitivity and specificity of 100% and 85%, respectively. In a large meta-analysis, Seshadri and colleagues concluded that SIS was as good as hysteroscopy in identifying all types of intracavitary uterine abnormalities, with a pooled sensitivity and specificity of 88% and 94%, respectively. Normal fallopian tubes are not detectable on ultrasound unless dilated; however, their patency can now be assessed using a contrast agent. This method was first introduced in 1984 by injecting fluid into the uterine cavity under ultrasound guidance and observing fluid in the cul-de-sac as proof of fallopian tube patency. Hysterosalpingo-contrast sonography (HyCoSy), which is a newly introduced technique, uses a mixture of saline solution with air as a contrast medium and can be performed using 2D or 3D ultrasound. , The injected air appears hyperechoic and can be followed during real-time ultrasound as it traverses the fallopian tubes and spills into the peritoneal cavity, proving tubal patency. Many different contrast agents have been developed and used in Europe but are not approved by the Food and Drug Administration for intrauterine use in the United States. , HyCoSy was found to have an accuracy equivalent to HSG when compared with the criterion standard of laparoscopic chromopertubation in the determination of tubal patency with sensitivities and specificities ranging between 75% and 96% and 67% and 100%, respectively. Malik and colleagues evaluated 30 women with primary and secondary infertility comparing the efficacy of HSG and sonosalpingography (SSG) in the assessment of tubal patency. They concluded that SSG was a safe and well-tolerated procedure and could be performed instead of HSG and laparoscopy. Despite HyCoSy being a promising newer imaging technique, a large majority of patients undergoing infertility workup are still being evaluated with HSG. This study remains an essential radiologic examination and often the most commonly chosen imaging modality to assess tubal patency and morphology as well as to evaluate the uterine cavity. The examination consists of the injection of water-soluble or oil-based contrast medium into the uterine cavity under fluoroscopic guidance through a small catheter. The decision to use water versus oil-based contrast to perform HSGs remains a debate, with a recent study showing that the use of the latter agent resulted in an increase in pregnancy rates and live births. HSGs are performed in the first half of the menstrual cycle, between days 7 and 12 when the endometrium is thin and also to avoid the possibility of a pregnancy. Spot radiographs of the filled uterine cavity and opacified tubes are obtained intermittently until free spillage of contrast into the peritoneal cavity is observed, confirming tubal patency. Roma Dalfó and colleagues evaluated 78 women with infertility using both HSG and hysteroscopy. They found that both tests had a high correlation, complementing each other in the diagnosis of uterine abnormalities with an overall 73% agreement. Phillips and colleagues reviewed the findings of 327 infertile women who were evaluated with TV ultrasound, hysteroscopy, and HSG and concluded that the latter was superior in the diagnosis of tubal abnormalities, particularly tubal obstruction. In their meta-analysis of 20 articles, Swart and colleagues came to the same conclusion, finding that HSG was a valuable diagnostic tool for diagnosing tubal obstruction. More recently, newer advanced imaging modalities have emerged using the 2D and 3D reconstruction capabilities of multidetector CT imaging and the 3D dynamic imaging capabilities of MR imaging to create virtual hysterosalpingograms. Both imaging techniques are based on the conventional technique for performing HSGs with the added advantages of evaluating the cervix, uterine cavity and its outside contour, the entirety of fallopian tubes including their patency, and the surrounding pelvic structures such as ovaries and adnexae. Postprocessing tools include a combination of volume rendering, multiplanar reformatting, maximum intensity projection, and virtual endoscopy, all of which require added postprocedure time and expertise.
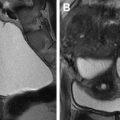
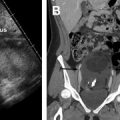
The role of MR imaging in the evaluation of female infertility is many folds: it is thought of as the gold-standard imaging modality for the diagnosis of MDAs; it is an essential imaging tool in the diagnosis and management of adenomyosis, deep infiltrative endometriosis, and leiomyomas, and is a great problem-solving examination when tubal and peritubal disease and adhesions are suspected on ultrasound or HSG. , In fact, Volondat and colleagues conducted a study comparing the accuracy of HSG and MR-HSG in 26 women with infertility. They found that MR-HSG had a sensitivity and specificity of 91.7% and 92.0%, respectively for the diagnosis of both tubal patency and intrauterine pathology. In addition, MR often plays a vital role in presurgical planning. Multiplanar reformatting from 3D acquisition allows for improved anatomic assessment and significantly reduced scan time. Unlike HSG, MR imaging is noninvasive and avoids the use of ionizing radiation. Unlike ultrasound, MR imaging grants a wider field of interrogation and is not limited by overlying bowel gas or patient body habitus and is usually a well-tolerated examination in the absence of claustrophobia. Standard pelvic MR imaging protocols include T1-weighted and T2-weighted images ( Table 2 ).
| Sequences | Plane | FOV, cm | Comment |
|---|---|---|---|
| T2W SSFSE | Coronal | 32–40 | General overview, assessment for concurrent anatomic anomalies (eg, renal) |
| 3D FS T2W | Coronal | 18–24 | Thin-cut volumetric acquisition, allows for multiplanar reconstruction to improved anatomic assessment, reduced scan time |
| GRE Dual-Echo T1W (In/Out) | Axial | 32–40 | Useful for visualization of blood products and characterization of adnexal pathology |
| EPI DWI | Axial | Targeted to region of interest | |
| 3D LAVAT1WFS Pre | Axial or Sagittal | 18–24 | Thin-cut volumetric acquisition |
| Optional | |||
| 3D LAVAT1WFS Post | Axial or Sagittal | 18–24 | Performed for additional characterization of any unrelated or unexpected finding seen on initial noncontrast evaluation |
| T2W FSE | Coronal | 18–24 | Performed for supplemental assessment of uterine anatomy |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree