Infertility, or subfertility, is the inability to achieve a clinical pregnancy after a 1-year period of regular unprotected sexual intercourse in women younger than 35 and after 6 months in women older than 35. Although initial assessment involves a multitude of factors, including a detailed medical history, physical examination, semen analysis, and hormonal evaluation, diagnostic imaging of the female partner often plays an important role in establishing the etiology for infertility. This article provides an overview of the multimodality imaging assessment of female infertility and details the developmental and acquired pelvic abnormalities in which diagnostic imaging aids in evaluation.
Key points
- •
Infertility can be primary or secondary and affects up to 12% of couples of reproductive-age worldwide.
- •
Causes of female infertility include congenital etiologies such as MDAs and acquired ones which range from ovulatory dysfunction to cervical factors and uterine and tubal abnormalities.
- •
The imaging investigation of female infertility consists of a multimodality approach, and includes a combination of primarily US and HSG, with MRI for problem-solving situations specifically if MDAs or deep infiltrative endometriosis are suspected.
- •
HSG remains the gold-standard imaging modality to assess tubal patency.
Congenital causes: müllerian duct anomalies
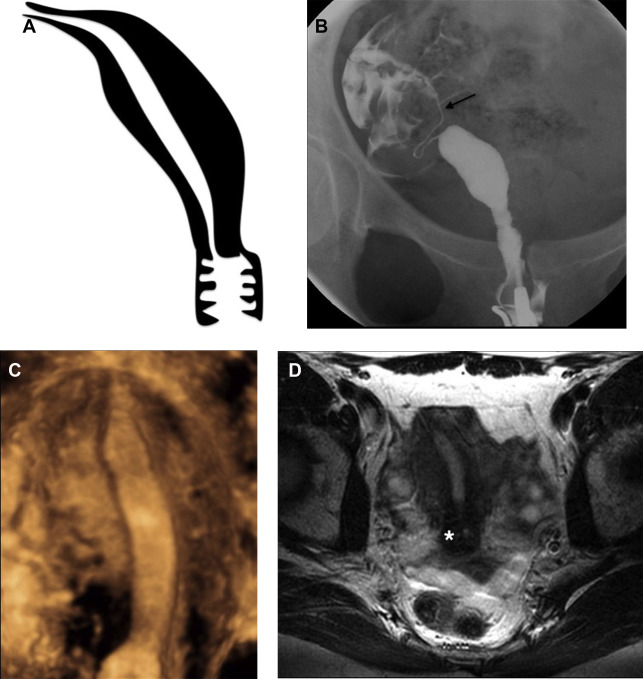
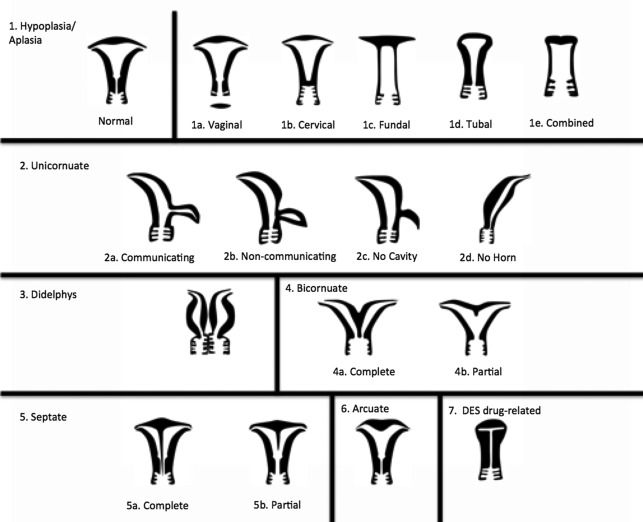
Müllerian duct anomalies (MDAs) represent a complex spectrum of congenital abnormalities resulting from failed development of the müllerian ducts, which normally give rise to the uterus, cervix, fallopian tubes, and upper third of the vagina ( Table 1 ). In keeping with the wide spectrum of anatomic appearances of MDAs, their presenting clinical features are also varied and include primary amenorrhea, endometriosis, spontaneous abortion, intrauterine growth restriction, and preterm labor. Although most women with MDAs have no issues conceiving, MDAs are associated with higher rates of spontaneous abortion, preterm delivery, and abnormal fetal lie. , In addition, although the overall prevalence of MDAs ranges from 1% to 5% in the general population, retrospective studies have shown that, among women with recurrent pregnancy loss, the prevalence of MDAs is substantially higher, ranging from 13% to 25%. Reproductive outcomes among patients with MDAs vary significantly by subtype. For example, 1 meta-analysis showed septate uterus to have the highest rate of spontaneous abortion (65%) of all uterine anomalies, compared with unicornuate uterus (50%), didelphys uterus (45%), and bicornuate uterus (30%). Multiple classification systems have been proposed to categorize MDA subtypes. , Although there is no universally accepted MDA classification system, the system proposed by the American Society for Reproductive Medicine (ASRM), formerly referred to as the American Fertility Society, remains the most widely used approach for categorization ( Fig. 1 ). This system subdivides MDAs into categories that show similar anatomic appearance, clinical manifestations, and treatment. , Of note, some complex MDAs do not fit neatly into an ASRM classification subtype. In these scenarios, it is important to describe each anomaly rather than attempt to categorize the MDA according to the dominant anatomic feature. In recent decades, magnetic resonance (MR) imaging has emerged as the imaging modality of choice to diagnose and classify MDAs, with reported accuracies of up to 100%.
| MDA Subtype | Embryologic Origin | Imaging Features | Important Considerations |
|---|---|---|---|
| Uterine agenesis/hypoplasia | Failure of müllerian duct proliferation | Spectrum of findings with most extreme form as complete absence of uterus, cervix, fallopian tubes, and upper vagina (MRKH syndrome) |
|
| Unicornuate uterus | Failure of müllerian duct development | Typically fusiform banana-shaped horn located off midline; with or without a small rudimentary horn |
|
| Bicornuate uterus | Failure of müllerian duct fusion | Two divergent communicating horns fused at the lower uterine segment; either with 1 (unicollis) or 2 cervices (bicollis) Deep external fundal cleft (>10 mm) |
|
| Uterus didelphys | Failure of müllerian duct fusion | Two divergent noncommunicating horns, each with its own cervix and (usually) proximal vagina |
|
| Septate uterus | Failure of septal resorption | Residual uterine septum, either partial (septum does not contact cervix) or complete (septum contacts cervix) Flat/convex external fundal cleft (<10 mm) Indentation depth > 10 mm |
|
| Arcuate uterus | Failure of septal resorption | Normal external fundal contour Broad-based smooth myometrial prominence at the internal fundal contour |
|
Uterine agenesis or hypoplasia
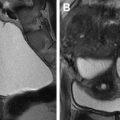
Accounting for approximately 5% to 10% of all MDAs, early developmental failure of the müllerian ducts results in complete agenesis or hypoplasia of the uterus, cervix, fallopian tube, and upper vagina. A wide spectrum of findings can be seen with this entity, with the most extreme (and most common) form showing complete absence of the uterus, cervix, fallopian tubes, and upper vagina known as Mayer-Rokitansky-Küster-Hauser syndrome. Because physiologic ovarian function is usually maintained (ovaries arise from the primitive yolk sac), patients show normal secondary sexual characteristics.
Typically, these patients present at the time of puberty with primary amenorrhea. MR imaging is ideal for further investigation because it can confidently differentiate uterine hypoplasia from agenesis as well as other causes of primary amenorrhea such as androgen insensitivity syndrome, which is seen in association with rudimentary testes. In addition, MR imaging allows evaluation for concurrent renal anomalies, which can be seen in up to 29% of patients with uterine agenesis. Sagittal T2-weighted imaging without fat saturation is the most useful sequence, showing absence of the uterus, cervix, and upper vagina in cases of complete agenesis ( Fig. 2 ). In the case of partial agenesis, a rudimentary uterus may be seen as a pelvic soft tissue mass of myometrial signal intensity with no zonal anatomy. It is important to describe the presence of a hypoplastic uterus, if present, because this can be associated with the presence of a uterine cavity, which can obstruct at the time of menarche leading to cyclic hematometra and/or endometriosis, possibly requiring surgical intervention. Patients with uterine agenesis or hypoplasia are invariably infertile and treatment goals in this population are primarily to enable normal sexual function, typically through the formation of a neovagina or lengthening of an existing distal vagina.
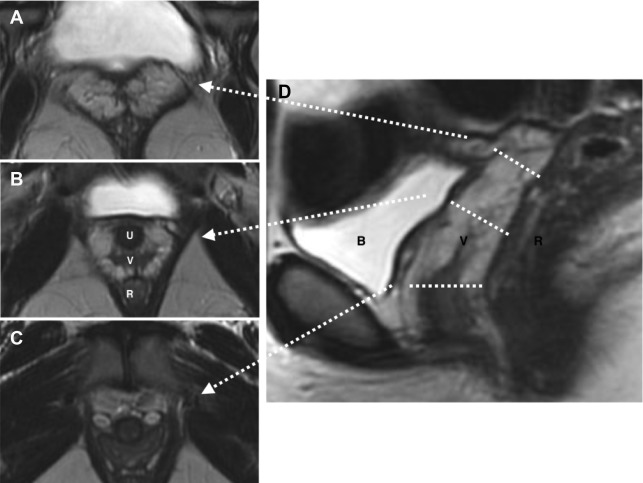
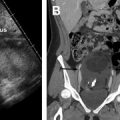
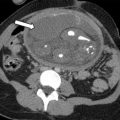
Unicornuate uterus
Accounting for approximately 20% of all MDAs, complete or near-complete arrested development of 1 müllerian duct with normal development of the contralateral duct results in unicornuate uterus. There are 4 subtypes commonly described, which relate to the potential presence of a rudimentary horn (RH) and its associated features: (1) communicating cavitary RH, (2) noncommunicating cavitary RH, (3) noncavitary RH, and (4) no RH. Approximately half of the cases of unicornuate uterus show a cavitary RH and, of those cases, most (70%) do not communicate with the contralateral (normal) uterine horn. This scenario may manifest clinically as cyclic pelvic pain caused by an increased prevalence of endometriosis related to retrograde flow during menses in the setting of an obstructed and distended RH. , , MR imaging is the preferred modality to assess a unicornuate uterus, which appears as a fusiform banana-shaped horn located off the midline ( Fig. 3 ). It is also the most sensitive imaging modality to detect the presence of an RH, which has a varied appearance by subtype ( Fig. 4 ). If there is no endometrium present within the RH, zonal anatomy is absent. When a cavitary RH is present, normal zonal anatomy is typically preserved and is best appreciated on T2-weighted sequences. In such instances, it is important to comment on such findings because surgical removal is often necessary to prevent symptoms related to obstruction and/or endometriosis. In addition, resection should be performed to prevent potential complications related to pregnancy occurring within the RH, which carries an increased risk of spontaneous abortion (up to 50% of cases); preterm labor; and, most significantly, uterine rupture. , , Ultrasonography (US) diagnosis of a unicornuate uterus and its variants can be challenging unless the clinician notices the smaller size or deviation of the uterus off midline or the presence of an adjacent smaller RH (see Fig. 4 ). Using three-dimensional (3D) US should help confirm the diagnosis by showing its characteristic banana shape and better visualize an RH if present. Unicornuate uterus has the strongest association with renal anomalies, occurring in up to 40% of cases, and classically occurs ipsilateral to the absent or rudimentary horn. Although the single most common renal anomaly in these patients is unilateral renal agenesis, other anomalies, such as duplicated collecting system, horseshoe kidney, ectopic kidney, and cystic renal dysplasia, have also been described. , , Given this association, MR examinations protocoled for the evaluation of suspected unicornuate uterus should include at least cursory views of the upper abdomen (eg, coronal single-shot fast spin echo) to evaluate for the presence of renal anomalies.