This article is an update and literature review of the clinical and neuroimaging findings of the commonly known rickettsial, spirochetal, and eukaryotic parasitic infections. Being familiar with clinical presentation and imaging findings of these infections is crucial for early diagnosis and treatment especially in patients who live in or have a travel history to endemic regions or are immunocompromised.
- •
Rickettsial, spirochetal and parasitic central nervous system (CNS) infections, although generally quite rare, are still being encountered in endemic regions around the world.
- •
Toxoplasmosis and neurosyphilis may manifest as opportunistic infections in immunocompromised patients, particularly in patients infected with human immunodeficiency virus.
- •
CNS involvement in spirochetal infections, particularly in neurosyphilis and Lyme disease, is quite varied in clinical presentation and imaging findings.
- •
A high index of suspicion and knowledge of the imaging features may lead to early diagnosis of the rickettsial, spirochetal, and parasitic CNS infections.
Rickettsiae
The Rickettsiae are small gram-negative bacteria, which are generally obligate intracellular parasites and transmitted to humans by arthropods. The exception is Coxiella burnetii, which is transmitted by inhalation and has an endospore form that is capable of surviving in an extracellular environment. The most common rickettsial infections are Rocky Mountain spotted fever, epidemic typhus, and Q fever. Rickettsial infections except for Q fever typically present with fever, rashes, and vasculitis. Imaging findings of central nervous system (CNS) involvement in Rocky Mountain spotted fever reported to date include diffuse cerebral edema, meningeal enhancement, arterial infarction, and signal abnormality within the distribution of perivascular spaces, which are dissimilar to the documented neuroimaging findings of Q fever consisting of (meningo) encephalitis, acute cerebellitis, and transverse myelitis. To the authors’ knowledge, there are no existing reports documenting neuroimaging findings of epidemic typhus or more commonly referred to as simply typhus. It should not be confused with typhoid fever, also known as typhoid, caused by the bacterium Salmonella typhi.
Owing to the small size of the bacteria, direct microscopic visualization of Rickettsiae is difficult and requires special stains such as Giemsa or Gimenez. Hence, serologic tests such as complement fixation test, indirect immunofluorescence, latex agglutination, and enzyme immunoassay tests, are commercially available and are used in the diagnosis of Rickettsial disease. Tetracyclines and chloramphenicol are effective in treatment, whereas sulfonamides enhance the disease and are contraindicated.
Rocky Mountain Spotted Fever
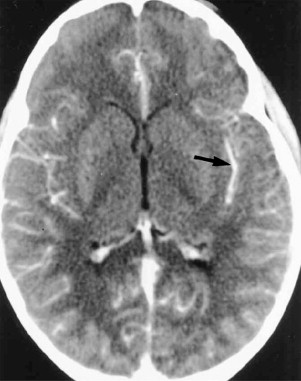
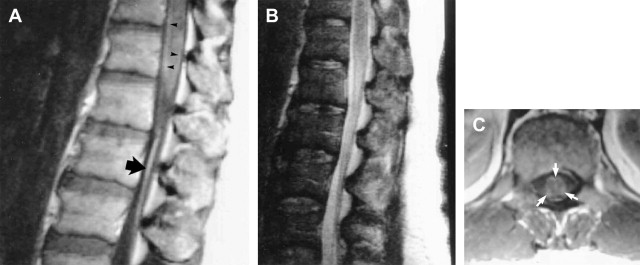
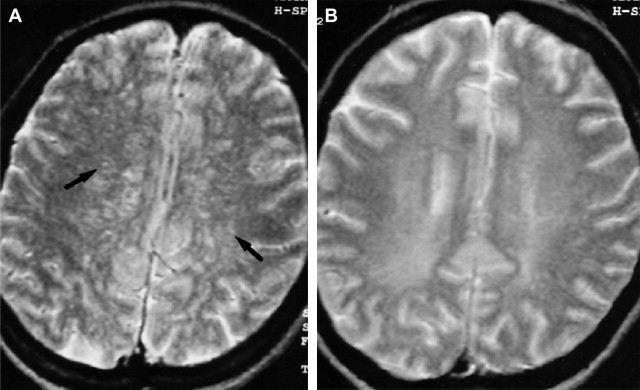
The causative agent in Rocky Mountain spotted fever is Rickettsia rickettsii, which is transferred to humans by the dog or wood tick bite. As opposed to the name that implies a mountainous predisposition, approximately 25% of the cases occur in North Carolina and more than 50% of the cases were reported in the South Atlantic region of the United States. Rickettsia rickettsii spreads via the blood stream, proliferates, and injures endothelial and vascular smooth muscle cells, thereby causing damage to the microcirculation of virtually all organs. Vascular lesions are prominent in the skin explaining the pathophysiologic basis for the rash found in Rocky Mountain spotted fever. The skin rash initially appears on the wrists, ankles, soles, and palms and later spreads to the trunk. The maculopapular skin lesions, later in the disease, may become petechial and eventually purpuric and ecchymotic and can rarely lead to skin necrosis or gangrene. Other clinical manifestations include fever, headache, myalgias, confusion, meningismus, ataxia, aphasia, paralysis, seizures, and coma. In the brain, vasculitis forms a characteristic pathologic appearance called typhus nodules, which are essentially perivascular infiltrates of lymphocytes, polymorphonuclear leukocytes, and macrophages. Other pathologic lesions include white matter microinfarctions and a predominantly mononuclear cell leptomeningitis. Early recognition of the CNS involvement is critical, because such involvement often causes increased mortality. Rocky Mountain spotted fever is still the most frequently reported life-threatening tick-borne infection, with mortality rates ranging from 2% to 10% even under adequate antibiotic therapy. The largest case series reported in the literature was contributed by Bonawitz and colleagues and reported the associated neuroimaging findings in patients with Rocky Mountain spotted fever. Of 34 patients, 28 had computed tomography (CT) of the brain (44 studies, 10 after contrast administration), 6 had contrast-enhanced brain magnetic resonance (MR) imaging, and 4 had both CT and MR studies of the brain. Four patients demonstrated abnormalities on CT imaging, including focal arterial basal ganglia infarction, diffuse cerebral edema, and diffuse meningeal enhancement ( Fig. 1 ). MR imaging was abnormal in 4 patients with findings consisting of focal basal ganglia and frontal lobe arterial infarctions, diffuse edema, diffuse meningeal enhancement, and prominent perivascular spaces in the region of the basal ganglia. In addition, one patient had thoracolumbar contrast-enhanced spine MR imaging, which depicted abnormal enhancement along the surfaces of the distal spinal cord as well as enhancement of the cauda equina ( Fig. 2 ). Although the presence of abnormal neuroimaging is rare, its presence is associated with worse clinical prognosis. However, the neuroimaging findings are potentially reversible if appropriate treatment is initiated early in the course of the disease. Total resolution of MR imaging findings was also shown in a single case by Baganz and colleagues in which there was reversal of extensive T2 signal abnormality in the distribution of the perivascular spaces without accompanying abnormal enhancement. Although nonspecific and also reported in diseases such as cryptococcosis and Lyme disease, the investigators postulated perivascular signal abnormality may reflect the underlying pathophysiology of Rocky Mountain spotted fever ( Fig. 3 ).
Epidemic Typhus
The causative agent of epidemic typhus is Rickettsia prowazekii, which is transmitted to humans via the louse. Similar to Rocky Mountain spotted fever, Rickettsia prowazekii has tropism to endothelial cells inducing clotting, which can lead to gangrene of the hands and feet. The disease is characterized by fever, headache, and a rash sparing the palms, soles, and face. This disease is typically seen in an epidemic setting especially in overcrowded populations living in poverty and unsanitary conditions. The disease is still considered to be a major threat by public health authorities, despite the efficacy of antibiotics, because poor sanitary conditions are conducive to louse proliferation. Previously Rickettsia prowazekii, the causal agent, was thought to be confined to human beings and their body lice; however, in 1975, Rickettsia prowazekii infection in human beings was observed due to contact with the flying squirrel Glaucomys volans in the United States.
Q Fever
This disease is recognized around the world and occurs mainly in people who come into contact with goats, sheep, and dairy cattle. Coxiella burnetii, the organism of Q fever, possesses unique characteristics within the Rickettsiae family such as the ability to form an endospore that is resistant to heating and drying and does not need a vector to be transmitted to humans. Transmission results from inhalation of dust contaminated with Coxiella burnetii from placenta, dried feces, urine, or milk or from aerosols in slaughterhouses. This disease resembles influenza, nonbacterial pneumonia, endocarditis, hepatitis, and encephalopathy rather than typhus. CNS involvement is a rare manifestation documented in a few reports of (meningo) encephalitis that occurred in the late course of acute Q fever. Sawaishi and colleagues reported an 8-year-old child presenting with a 1-week history of moderate fever and headache and found to have acute cerebellitis on MR imaging with swelling and diffuse T2 signal abnormality of a cerebellar hemisphere. Serologic tests revealed positive titers for Q fever. The acute imaging findings resolved following treatment with only residual parenchymal volume loss of the affected cerebellar hemisphere. Sempere and colleagues documented a case of Q fever encephalitis involving the temporal lobe, which created confusion at initial presentation with resemblance to herpes simplex encephalitis. In addition, there are a few reports in the literature concluding that infection with Coxiella burnetii can be associated with acute transverse myelitis as a result of direct infectious or parainfectious pathogenic mechanisms. This central nervous manifestation of Coxiella burnetii adds to the treatable causes of acute myelitis and should therefore be considered as a differential diagnosis, especially in areas endemic for Q fever. Moreover, in a study by Garron and colleagues, Coxiella burnetii was amongst the causative agents for nontuberculous spondylodiscitis in children in whom MR imaging and needle aspiration of the disc aided in diagnosis.
Spirochetes
Spirochetes are a phylum of gram-negative bacteria, which are distinguished by the location of their flagella that run lengthwise between the cell wall and outer membrane. These flagella cause a spiral motion that facilitates movement. Three genera whose members are human pathogens are Treponema, Borrelia, and Leptospira. Syphilis and Lyme disease, which are caused by Treponema pallidum and Borrelia burgdorferi, respectively, are the most commonly encountered diseases in this pathogen group. Both diseases present in the form of well-recognizable clinical stages usually beginning with an initial skin lesion. However, CNS involvement in both diseases is quite varied in clinical presentation and imaging findings. In addition, imaging may be normal in both diseases even in the setting of known CNS involvement with evidence of positive cerebral spinal fluid (CSF) tests. Neurosyphilis may present with numerous findings including: meningeal and cranial nerve enhancement, arteritis and ischemia, mass lesions known as gummas, cortical atrophy, hydrocephalus, and mesial temporal signal abnormality mimicking herpes simplex encephalitis, mesial temporal sclerosis, and paraneoplastic limbic encephalitis. Hence, it is called “the great imitator”. Similarly, Lyme disease also acts as an imitator with various clinical and radiologic presentations. Imaging findings of CNS involvement of Lyme disease are also myriad including, meningeal and cranial nerve enhancement, white matter signal abnormality occasionally resembling multiple sclerosis, prominent Virchow-Robin spaces, enhancing masslike parenchymal lesions, arteritis, ischemia, hemorrhage, meningoradiculitis, acute transverse myelitis, and ocular myositis.
Syphilis
Syphilis is a chronic venereal disease with multiple presentations. The causative spirochete, Treponema pallidum is a gram-negative corkscrew-shaped bacterium, which is too slender to be seen on Gram stain, but can be visualized by silver stains, dark-field examination, and immunofluorescence techniques. Sexual contact is the usual mode of spread. However, there may be nonsexual transmission via skin contact with an infected skin ulcer. Transplacental transmission of Treponema pallidum occurs readily, and active disease during pregnancy results in congenital syphilis.
Syphilis is a chronic infection with 3 well-characterized stages. Cases of syphilis surged upward in the setting of AIDS. Primary syphilis is characterized by a chancre (sore or ulcer) at the site of inoculation and painless regional lymphadenopathy. If left untreated, a secondary stage manifests during which a maculopapular rash is seen on the palms of the hand and soles of the feet with hematological dissemination of the bacteria. This rash is usually seen 2 to 8 weeks after the chancre appears, but in AIDS patients, there is much more rapid progression to secondary syphilis. About one-third of patients with secondary syphilis progress to tertiary syphilis and approximately one-third of those develop neurosyphilis. The tertiary stage usually occurs after a latent period of at least 5 years and has 3 main manifestations: cardiovascular syphilis, neurosyphilis, and syphilitic gummas. Cardiovascular disease is usually in the form of luetic aortitis which becomes apparent as aortic valve insufficiency and aneurysms of the proximal aorta. Gummas are nodular lesions likely related to the development of delayed hypersensitivity to the bacteria. These lesions may occur in various sites, most commonly in bone, skin, and the mucous membranes of the upper airway and mouth.
Although neurosyphilis may appear at any stage of systemic infection, more commonly it occurs at late stages, primarily the tertiary stage. CNS involvement can be asymptomatic, which accounts for about one-third of neurosyphilis cases and detected when CSF tests are abnormal with increased white cell counts, especially lymphocytes, increased protein, and decreased glucose. Although symptomatic neurosyphilis may manifest in several ways, in general, 2 major clinical subtypes are recognized: (1) meningovascular disease and (2) parenchymal disease.
Meningeal neurosyphilis usually appears within the first 2 years of infection and may result in headache, meningeal signs, and cranial nerve palsies, most frequently involving cranial nerves VII and VIII ( Fig. 4 ). Pathologically, there is widespread thickening of the meninges, meningeal lymphocytic infiltrates, and perivascular lymphocytic infiltrates. Meningeal enhancement may be present and is usually better seen on MR imaging than CT. Hydrocephalus may also occur. Involvement of cranial nerves VII and VIII in syphilitic basilar meningitis presents with facial paralysis, sensorineural hearing loss, and vertigo and radiologically, with enhancement of the seventh and eighth cranial nerves and cochlea as well as the meninges lining the internal auditory canals. Involvement of the optic, oculomotor, and abducens nerves has also been reported ( Fig. 5 ).





