Neurocysticercosis (NCC) is an infection of the central nervous system by the Taenia solium larvae, and is the most common cause of acquired epilepsy in endemic regions. The natural history of parenchymal NCC lesions can be divided into 4 stages with unique imaging and clinical features. Evaluation of cysticerci is challenging on conventional magnetic resonance (MR) imaging and computed tomography, and is significantly improved with MR cysternography techniques. Differentiation of NCC lesions from metastatic disease and pyogenic abscesses can be improved with advanced MR imaging including 1 H nuclear MR spectroscopy, diffusion-weighted imaging, and MR perfusion imaging.
- •
Neurocysticercosis (NCC) is the most common cause of acquired epilepsy in endemic regions.
- •
Parenchymal NCC lesions can be divided into 4 stages with unique imaging and clinical features.
- •
Intraventricular and subarachnoid NCC forms have a higher risk of complications.
- •
Evaluation of intraventricular and subarachnoid NCC is improved with magnetic resonance (MR) cysternography techniques.
- •
Advanced MR imaging can be helpful in differentiating of NCC lesions from metastatic disease and pyogenic abscesses.
Introduction
Neurocysticercosis is the result of the implantation of the cestode Taenia solium (pork tapeworm) in the human host. The larval stage of this organism may become disseminated throughout the central nervous system (CNS) of the affected individual. Neurocysticercosis is one of the most common causes of epilepsy in endemic regions throughout the world as well as in United States and European immigrant populations from these regions. Endemic regions include Latin America, parts of Oceania, Asia, Eastern Europe, and Africa. For a more thorough understanding of this disease, a full discussion of the complex life cycle of this parasite is necessary.
Life cycle and pathogenesis
The T solium life cycle includes humans as the definitive hosts and pigs as intermediate hosts. Sequential infection of both hosts is needed to complete the reproductive cycle of the tapeworm.
T solium is typically acquired by the human (definitive host) by ingestion of poorly cooked pork containing the larval form of the organism (cysticerci). The ingested larvae emerge in the gastrointestinal tract. The organism’s head, known as the scolex, implants in the mucosa of the small intestine and the tapeworm grows by generating segments known as proglottids. The adult tapeworm may reach up to 4 m in length and has hundreds of proglottids. The oldest and most distal proglottids contain tens of thousands of mature eggs ready to infect a new host. These proglottids are shed and excreted in the human feces. The intestinal infection by this parasite is referred to as taeniasis, which in many cases is asymptomatic; however, it may occasionally cause mild diarrhea and abdominal pain. Consequently, many individuals with taeniasis are not aware of their disease.
The pig (intermediate host) typically acquires the parasite by injection of feed contaminated with human fecal material containing T solium eggs (oncospheres). The larval forms of the parasite emerge from the eggs in the gastrointestinal tract, penetrate the wall of the bowel, and disseminate via the bloodstream throughout the muscle tissue of the pig, forming cysts in the surrounding tissue, known as cysticerci.
However, occasionally humans may become the intermediate hosts instead, which is manifested as cysticercosis. When humans ingest food contaminated with human feces containing T solium eggs, the larvae similarly invade the bloodstream of the humans and disseminate throughout the tissues of the body, forming cysticerci at terminal arterioles within the CNS, subcutaneous tissues, muscles, and so forth.
Neurocysticercosis refers specifically to CNS involvement by cysticercosis. Although involvement of muscle and subcutaneous tissues by this disease is frequently asymptomatic, within the CNS cysticerci can produce a variety of clinical neurologic symptoms.
Initially, the tapeworm larvae invade the CNS and form viable cysticerci with minimal host-mediated inflammatory reaction in the surrounding brain parenchyma. Presumably, the blood-brain barrier and factors secreted by cysticerci inhibit a significant immune response to the lesion within the CNS.
Degeneration of the cyst may occur in several months to years with associated inflammatory response and edema, which can result in clinical symptoms. It is thought that degeneration of the cyst occurs when the larva fails to inhibit the immune response. The cyst may rupture, releasing antigens into surrounding tissue and resulting in more severe inflammation. The severity of associated inflammatory response and associated clinical manifestations vary extensively in each case.
Cysticerci may also involve the subarachnoid space of the head and spine. Rarely, cysticerci may involve the spinal cord parenchyma.
The most common clinical manifestation of neurocysticercosis is seizure. In addition, cysticerci may cause obstructive hydrocephalus, intracranial hypertension, mass effect, and cerebral infarction. An acute encephalitis-like presentation has been described in the pediatric population, which is thought to represent a severe inflammatory response to cysticercal involvement of the CNS.
Life cycle and pathogenesis
The T solium life cycle includes humans as the definitive hosts and pigs as intermediate hosts. Sequential infection of both hosts is needed to complete the reproductive cycle of the tapeworm.
T solium is typically acquired by the human (definitive host) by ingestion of poorly cooked pork containing the larval form of the organism (cysticerci). The ingested larvae emerge in the gastrointestinal tract. The organism’s head, known as the scolex, implants in the mucosa of the small intestine and the tapeworm grows by generating segments known as proglottids. The adult tapeworm may reach up to 4 m in length and has hundreds of proglottids. The oldest and most distal proglottids contain tens of thousands of mature eggs ready to infect a new host. These proglottids are shed and excreted in the human feces. The intestinal infection by this parasite is referred to as taeniasis, which in many cases is asymptomatic; however, it may occasionally cause mild diarrhea and abdominal pain. Consequently, many individuals with taeniasis are not aware of their disease.
The pig (intermediate host) typically acquires the parasite by injection of feed contaminated with human fecal material containing T solium eggs (oncospheres). The larval forms of the parasite emerge from the eggs in the gastrointestinal tract, penetrate the wall of the bowel, and disseminate via the bloodstream throughout the muscle tissue of the pig, forming cysts in the surrounding tissue, known as cysticerci.
However, occasionally humans may become the intermediate hosts instead, which is manifested as cysticercosis. When humans ingest food contaminated with human feces containing T solium eggs, the larvae similarly invade the bloodstream of the humans and disseminate throughout the tissues of the body, forming cysticerci at terminal arterioles within the CNS, subcutaneous tissues, muscles, and so forth.
Neurocysticercosis refers specifically to CNS involvement by cysticercosis. Although involvement of muscle and subcutaneous tissues by this disease is frequently asymptomatic, within the CNS cysticerci can produce a variety of clinical neurologic symptoms.
Initially, the tapeworm larvae invade the CNS and form viable cysticerci with minimal host-mediated inflammatory reaction in the surrounding brain parenchyma. Presumably, the blood-brain barrier and factors secreted by cysticerci inhibit a significant immune response to the lesion within the CNS.
Degeneration of the cyst may occur in several months to years with associated inflammatory response and edema, which can result in clinical symptoms. It is thought that degeneration of the cyst occurs when the larva fails to inhibit the immune response. The cyst may rupture, releasing antigens into surrounding tissue and resulting in more severe inflammation. The severity of associated inflammatory response and associated clinical manifestations vary extensively in each case.
Cysticerci may also involve the subarachnoid space of the head and spine. Rarely, cysticerci may involve the spinal cord parenchyma.
The most common clinical manifestation of neurocysticercosis is seizure. In addition, cysticerci may cause obstructive hydrocephalus, intracranial hypertension, mass effect, and cerebral infarction. An acute encephalitis-like presentation has been described in the pediatric population, which is thought to represent a severe inflammatory response to cysticercal involvement of the CNS.
Diagnosis of neurocysticercosis
The clinical presentation of neurocysticercosis is often nonspecific and highly variable, depending on the number and location of the lesions. This variation may delay the correct diagnosis. Serologic tests are used to establish the diagnosis in suspected cases, including enzyme-linked immunoelectrodiffusion transfer blot (EITB). EITB has been used with different antigens and has demonstrated very high sensitivity and specificity for cysticercosis. Imaging plays a critical role in confirming and fully characterizing CNS involvement by cysticercosis.
Parenchymal neurocysticercosis
Neurocysticercosis most commonly manifests in the parenchyma of the brain and typically involves the cerebral hemispheres, with lesions commonly found at the gray matter–white matter junction, presumably resulting from deposition of the larvae in terminal small vessels of this region. Multiple lesions are usually present, although the number is highly variable, with solitary lesions as well as innumerable lesions in a miliary pattern occasionally encountered. Cerebellar, basal ganglia, or brainstem lesions may also be found in individuals with numerous lesions.
Computed tomography (CT) and magnetic resonance (MR) imaging excel at detecting acute and chronic forms of neurocysticercosis and its complications. Acute symptomatic lesions are best visualized on contrast-enhanced MR imaging. However, chronic lesions are best visualized on noncontrast CT as hyperdense calcifications, because MR imaging signal voids associated with chronic calcified lesions are difficult to identify on all sequences.
The earliest form of larval invasion is noncystic and is usually not detectable on imaging, because of frequent lack of edema at that stage. However, occasionally lesions may develop associated edema and focal enhancement before cystic transformation.
Four stages of neurocysticercosis lesions can be recognized on CT and MR imaging as they naturally evolve from the acute to the chronic form.
Vesicular Stage
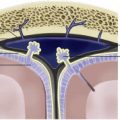
Within a few weeks the larva transforms into a cysticercal cyst containing an invaginated scolex, representing a viable, active form of cysticercosis. The cyst matures over the course of several months and may subsequently enlarge to approximately 1 cm. Imaging hallmarks of this stage include visualization of the scolex within a cyst and absence of enhancement or thin linear enhancement of the cyst wall. The cyst fluid usually demonstrates the same signal intensity as cerebrospinal fluid (CSF) on all MR imaging sequences. On CT the cyst demonstrates attenuation, similar to the CSF ( Fig. 1 ).
The scolex is identifiable in some lesions at this stage, as it has variable signal characteristics and may enhance ( Fig. 2 ). Fluid-attenuated inversion recovery (FLAIR) imaging frequently improves the visualization of the T2-weighted hyperintense scolex immersed in CSF-like fluid of the cyst.
Colloidal Vesicular Stage
As already described, the cyst usually begins to degenerate in several months. Early degeneration of the cysticercus results in destruction of the scolex, development of proteinaceous cyst contents, and associated inflammatory response in the surrounding brain parenchyma. This process represents a degenerating, active form of cysticercosis.
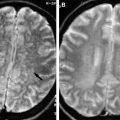
The process is manifest on CT, with hyperdense fluid within the cyst and ring enhancement of the lesion. MR imaging frequently reveals T1 hyperintensity of the cyst fluid and lack of a scolex. T1-weighted postcontrast images also reveal a ring-enhancement pattern. T2-weighted images frequently demonstrate adjacent edema and may show a rim of the cyst of low signal intensity ( Fig. 3 ). However, absence of diffusion restriction is evident on diffusion-weighted (DW) imaging, helping to differentiate this lesion from an abscess ( Fig. 4 ). Occasionally a fluid-fluid level may be evident within, suggestive of internal debris.
Granular Nodular Stage
As degeneration of the cysticercus progresses, the cyst decreases in size and transforms into a smaller nodular lesion. This process represents a degenerating, active form of cysticercosis. CT and MR imaging demonstrate enhancement of the nodular lesion or a small ring-enhancing lesion at this stage. Mild associated edema may be identified in adjacent brain parenchyma ( Fig. 5 ).
Nodular Calcified Stage
The nodular calcified stage is the end stage of cysticercal degeneration, with transformation into a small calcified granulomatous lesion. This process represents a degenerated, inactive form of cysticercosis. Usually no associated edema or enhancement is observed, representing a lack of immune response to this end-stage, nonviable lesion. CT is most sensitive for these lesions, revealing them as hyperdense parenchymal lesions ( Fig. 6 ). The lesions are hypointense on T1- and T2-weighted imaging, and may be difficult to identify ( Fig. 7 ).