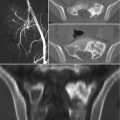
Fig. 5.1
Sacral chordoma in a 55-year-old woman. (a, b) Axial and sagittal CT scan. Large osteolytic lesion arising in the midline from the lower sacrum. The mass shows low attenuation and some internal calcifications. (c) Sagittal T1-w MRI, (d) oblique coronal T-2 weighted MRI: the lesion has a low signal intensity on T1-w images and a high signal intensity on T2-w images; it extends in the spinal canal and in the presacral space
Septations can be present and appear as hypointense on T2-w images. Small foci of low signal intensity on T2-w images can represent hemosiderin deposits from previous hemorrhage [11].
The main differential diagnosis of sacral chordoma include chondrosarcoma, benign notochordal cell tumor (BNCT), giant cell tumor, Plasmacytoma, myxopapillary ependymoma, and metastases.
The characteristic signs in favor of chordoma are its usually midline position, situated very low in the sacrum (S3 to S5) and extended upward in the sacral canal.
Chondrosarcoma constitutes the major differential diagnosis, especially in the presence of tumor calcifications, often observed in chondroid chordomas. The location (chondrosarcoma arise off midline, from the sacro-iliac cartilage), a heterogeneous signal intensity on T2-w images, the lack of areas of hemorrhage within the tumor, and septations contrast enhancement may help in the diagnosis of chondrosacroma [12]. Diffusion weighted-MRI can be useful in differential diagnosis since ADC values seem to be higher in chondrosarcoma than in chordoma [13].
Chordoma should also be distinguished from Benign Notochordal Cell Tumor (BNCT), a benign lesion arising from notochord remnants (previously called giant “notochordal rests” or “notochordal hamartomas”) and believed to be rarely a precursor of chordoma. It is very frequent on autopsies (20% of the population) but rare on imaging exams (probably because not enough large to be detected) [14]. On radiographs, BNCT are often invisible or can show a vague sclerosis. CT shows a sclerotic lesion without cortical disruption or expansion. On MRI, BNCT are hyperintense on T2-w images and hypointense on T1-weighted images without any contrast enhancement (Fig. 5.2). Unlike chordoma, no soft tissue component is present [15–17]. If radiological characteristics are typical of BNCT, biopsy is not necessary unless the lesion changes; follow-up is recommended.
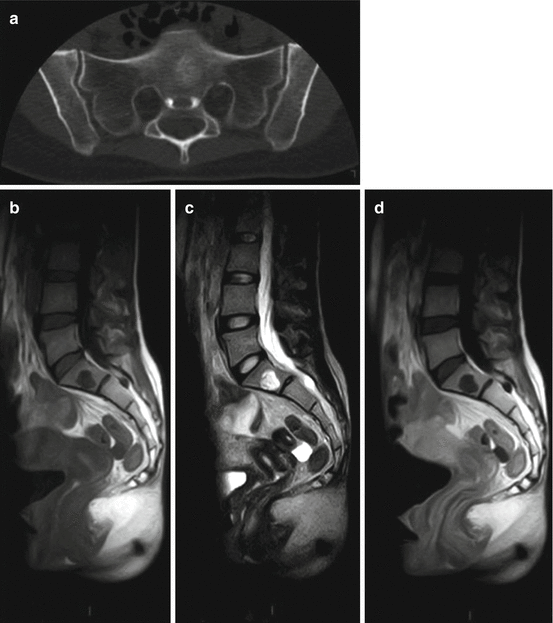

Fig. 5.2
Benign notochordal cell tumor (BNCT) in a 31-year-old woman: (a) axial CT scan shows a sclerotic lesion in S1. (b) Sagittal T1-w MRI, (c) sagittal T2-w MRI, (d) sagittal T1-w MRI after Gd: the lesion has a low signal intensity on T1-w images, is hyperintense on T2-w images, and shows no contrast enhancement
Giant cell tumors usually affect younger patients, have an eccentric and upper-sacral location, polycystic areas, fluid–fluid level, and frequently involve the sacro-iliac joint.
Myxopapillary ependymomas may show very similar characteristics to those of chondroma but they arise in the spinal canal rather than in the bone and usually demonstrate a more intense Gadolinium contrast enhancement.
After surgery, MRI follow-up of chordomas should be performed every 6 months for the first year after diagnosis to detect local recurrence or progression. The whole spine must be studied on MRI to detect leptomeningeal recurrences. Thereafter, if there is no progression, MRI is recommended yearly for at least 15 years [18].
5.2.5 Chondrosarcoma
Chondrosarcomas accounts for 7–12% of malignant primary tumor of the spine and are more common in the thoracic spine, while they are rare in the sacrum.
Primary chondrosarcoma of the sacrum predominantly affects patients between 30 and 70 years, with a male predominance (2-4:1) [19].
On radiographs and CT, chondrosarcoma appears as a destructive lytic lesion, usually eccentric in location, with a lobulated contour and endosteal scalloping. The lesion can be associated with a soft tissue mass [10].
On CT, the non-mineralized, cartilaginous portion of the tumor has a low density while the mineralized portion can show typical “ring and arc” calcifications.
On MRI, cartilaginous nodules are iso-hypointense on T1-w images and show lobular high intensity on T2-w images; calcifications are seen as areas of signal void on all MRI sequences (Fig. 5.3). Contrast enhancement is usually mild and can be either nodular, septal, or diffuse [1]. Dedifferentiation can be detected on CT or MRI in a part of the tumor taking up contrast medium strongly. The biopsy must include this suspicious location.
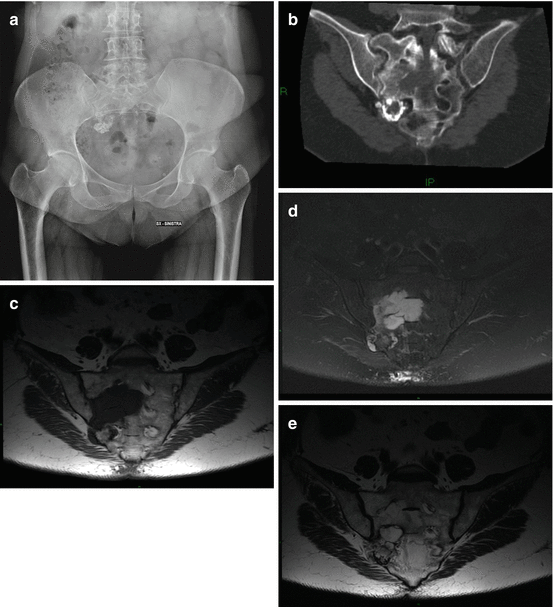

Fig. 5.3
Chondrosarcoma of the right sacral wing in a 60-year-old woman. (a) X-ray, (b) oblique coronal CT scan. “Ring and arc” calcifications in the osteolytic lesion (c) oblique coronal T1-w MRI image, (d) oblique coronal T2-w Fat Sat MRI, (e) oblique coronal T1-w MRI after Gd. The lesion is iso-hypointense on T1-w images and has a lobular high intensity on T2-w images
5.2.6 Ewing Sarcoma
Ewing sarcoma usually affects patients between the age of 10 and 30 years with a male predominance. More than one half of vertebral Ewing sarcoma arise in the sacrum (70% in the sacral wing) [20], but just 0.5% of all Ewing sarcoma involve this region.
These tumors typically fill the bone marrow cavity and destroy the cortex. The soft tissue mass associated to the tumor is often larger than the intra-osseous component.
Radiography and CT show a osteolytic destructive lesion, often associated with a sclerotic reaction [21]. CT better depicts the soft tissue involvement and the extension of the bony lesion.
MRI clearly depicts both intra- and extra-osseous components of the tumor, including paraspinal, extradural, and presacral involvement. MRI features are nonspecific; the lesion is iso-hypointense on T1-w and iso-hyperintense on T2-w images with variable contrast enhancement. Both CT and MRI are useful for staging of the tumor but are nonspecific because the osteolysis and the soft tissue mass can be limited and the lesion can be located either anterior or posterior to the spinal canal (Fig. 5.4).
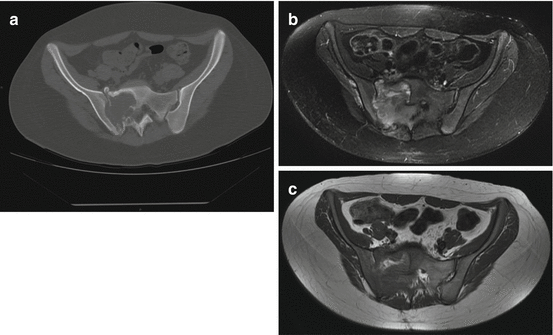

Fig. 5.4
Ewing sarcoma in a 20-year-old woman: (a) coronal CT, (b) axial T1-w MRI image, (c) axial T2-w Fat Sat MRI image. The lesion appears as a destructive osteolysis with a sclerotic reaction and invasion of right sacral foramina and sacro-iliac joint
5.2.7 Osteosarcoma
Sacral osteosarcoma is rare with only 1% of osteosarcoma involving the sacrum with a peak of incidence in the fourth decade [22].
Most sacral osteosarcomas are secondary, occurring after radiotherapy or Paget’s disease. The tumor has an aggressive, permeative osteolytic pattern with cortical disruption and soft tissue involvement. Tumor extension across the sacro-iliac joint and into the spinal canal is common [23]. On CT, most sacral osteosarcomas contain “cloud-like” osteoid mineralization [24]. MRI shows no specific features.
5.2.8 Other Malignant Bone Tumors
Fibrosarcomas of the sacrum are rare; they arise from a preexisting lesion in about ¼ of cases, usually previously irradiated bone, Paget’s disease, bone infarct or giant cell tumour, fibrous dysplasia, or ameloblastic fibroma.
Clear cell sarcoma (CCS) is a very rare tumor (1% of soft tissue sarcomas) that usually arises from tendons or aponeuroses of the extremities. It usually affects young patients (25–40 years), presenting with a slowly growing, painless mass [25, 26].
Few cases of clear cell sarcoma of the sacrum are reported. MR is the gold standard imaging method for the diagnosis of the CCS.
The lesion usually is hyperintense on T1-weighted images and hypointense on T2-weighted images due to the melanin content of sarcomatous cells [27].
Angiosarcoma, hemangiopericytoma, and pleomorphic sarcoma in the sacrum are exceptional.
5.3 Benign Bone Tumors
Ten percent of all benign tumors (pseudotumors) involve the sacrum. The most common are giant cell tumours (60% of cases) followed by aneurysmal cysts (4%) and osteoblastoma.
5.3.1 Giant Cell Tumour
Giant cell tumour (GCT) is the second most frequent primary tumor of the sacrum after chordoma; the peak of frequency is between 20 and 30 years with a female predominance (2/1).
Although usually benign, GCT are locally aggressive and 5–10% can undergo malignant transformation [28].
GCT usually involves the upper sacrum and has an eccentric location.
On CT, GCT appears as a well-demarcated polycyclic expansile lesion, inducing usually circumscribed osteolysis and rarely associated with a rim of sclerosis, classically with no calcifications.
The tumor is locally invasive, disrupting the cortex, and invading soft tissues. Crossing of the sacro-iliac joint is frequent (Figs. 5.5 and 5.6).
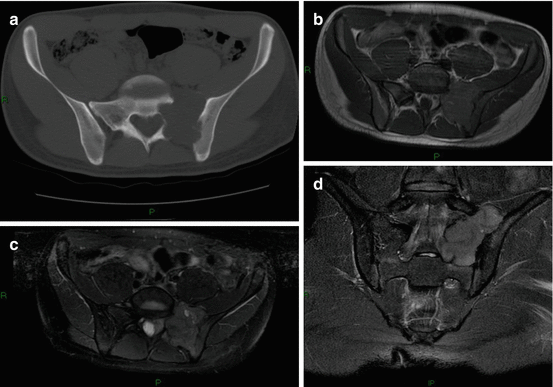
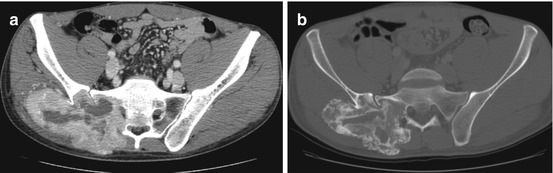

Fig. 5.5
Giant cell tumor (GCT) in a 30-year-old man: (a) axial CT scan shows an eccentric osteolytic lesion in the upper sacrum crossing the left sacro-iliac joint, (b) axial T1-w MRI, (c) axial T2-w FS image, (d) coronal T2-w FS image. On MRI, the lesion has a heterogeneous low to intermediate signal intensity on T1-w and on T2-w images

Fig. 5.6
Giant cell tumor in a 25-year-old man. Axial CT scan before (a) and after (b) treatment with Denosumab. The lesion is massively ossified. That indicates an efficient treatment, and must not be misdiagnosed as malignant transformation
MRI shows anterior and posterior extension of the mass with a heterogeneous signal both on T1 and T2-w images. Generally, the tumor has a low-to-intermediate signal intensity on T1-w images. On T2-w images, GCT is iso-hypointense to the normal spinal cord in 63–96% of cases [29]. This seems to be related to the relative collagen content of fibrous components and hemosiderin within the tumor. Although this feature is not unique to GCT, it can help in the differential diagnosis because most other spinal neoplasms (metastases, myeloma, lymphoma, and chordoma) show high signal intensity on T2-w images.
Evidence of hemorrhage (with high signal intensity on T1-w and T2-w sequences or T2-w hypointense foci for hemosiderin deposits) and necrosis in the center of the tumor, with fluid–fluid levels, are characteristic [30]. Secondary aneurismal bone cyst with fluid–fluid levels can be detected.
5.3.2 Aneurysmal Cyst
Aneurysmal cyst is a space-occupying lesion comprising blood-filled cysts and is rare in the sacrum (<5%). About 80% of aneurysmal cysts are discovered before the age of 20, with a slight female predominance (56%) [10].
On CT, it appears as a purely lytic lesion without solid components, surrounded by a thin calcified border. MRI shows a well-limited multiloculated lesion with a high signal intensity on T2-w images, associated with fluid–fluid levels and surrounded by a hypointense rim (Fig. 5.7). The lesion presents a strong contrast enhancement because it is highly vascularized.
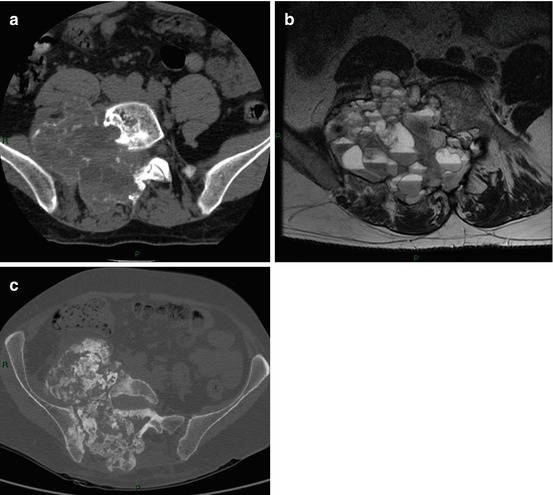

Fig. 5.7
Aneurismal bone cyst in an 18-year-old boy. (a) Axial CT scan (soft tissues window) depicts an osteolytic lesion in the right sacral wing surrounded by a thin sclerotic border. (b) Axial T2-w MRI: the lesion is multiloculated with fluid–fluid levels, (c) axial CT scan post-embolization (bone window) shows a sclerotic ossification within the cyst
No solid tissue component, and a T2-w hypointense rim, regularly hyperdense on CT in an expansile sacral lesion with fluid–fluid level in a young patient are typical features that suggest the diagnosis of aneurismal cyst. However, biopsy remains necessary especially for the differential diagnosis with GCT or rarely with telangiectatic osteosarcoma that may both present fluid–fluid levels [6].
5.3.3 Osteoid Osteoma
Osteoid osteoma rarely involves the sacrum (2%) but it represents 9% of all benign sacral tumors. Seventy-five percent of osteoma osteoid occur in patients before the age of 25 with a male predominance [21]. The classical symptom is dull pain, worse at night, and relieved by aspirin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree