The central skull base (CSB) constitutes a frontier between the extracranial head and neck and the middle cranial fossa. The anatomy of this region is complex, containing most of the bony foramina and canals of the skull base traversed by several neurovascular structures that can act as routes of spread for pathologic processes. Lesions affecting the CSB can be intrinsic to its bony-cartilaginous components; can arise from above, within the intracranial compartment; or can arise from below, within the extracranial head and neck. Crosssectional imaging is indispensable in the diagnosis, treatment planning, and follow-up of patients with CSB lesions. This review focuses on a systematic approach to this region based on an anatomic division that takes into account the major tissue constituents of the CSB.
The central skull base (CSB), the ultimate frontier between the intracranial compartment and the extracranial head and neck, can be affected by intrinsic lesions originating from the bony-cartilaginous structures of the skull base proper or by lesions originating from the neighboring structures from the intracranial compartment above or from the extracranial head and neck below. Because clinical assessment of the CSB is limited, cross-sectional imaging has become the mainstay for the diagnosis, treatment planning, and follow-up of patients with skull base lesions. Developments in cross-sectional imaging and in surgical and targeted radiation therapy techniques have largely contributed to improve the prognosis of patients with skull base tumors and to decrease the surgical-related morbidity and mortality. Multidisciplinary skull base teams, including ear, nose, and throat (ENT) surgeons; neurosurgeons; radiation therapists; radiologists; and oncologists, have been created to provide a comprehensive approach to patients with skull base lesions with the aim of maximizing the chances for long-term survival with the minimum amount of dysfunction and disfigurement possible.
Cross-sectional imaging can narrow down the differential diagnoses according to the site of origin, pattern of growth, and imaging features of a given lesion; can accurately delineate tumor margins; and can determine the precise relations between the lesion and important surrounding structures.
This review focuses on the contribution of imaging to patient management, providing a systematic approach to CSB lesions based on an anatomic division of the CSB and on the tissue constituents present in each division. Detailed knowledge of skull base anatomy is required for correct imaging diagnosis and for accurate delineation of lesions.
Anatomy
Viewed from above, the skull base shows three naturally contoured regions that grossly correspond to the anterior skull base, CSB, and posterior skull base. The CSB makes up the floor of the middle cranial fossa and is mainly composed of the sphenoid and temporal bone anterior to the petrous ridge ( Fig. 1 ). It is separated from the anterior skull base by a line that follows the tuberculum sella, the anterior clinoid processes, the posterior margin of the lesser sphenoid wings, and the anterior and superior rim of the greater sphenoid wings and is separated from the posterior skull base by a line following the spheno-occipital synchondrosis medially and the petroclival synchondrosis and superior ridge of the petrous and mastoid bones posteriorly and laterally.
Imaging approach to pathologic findings
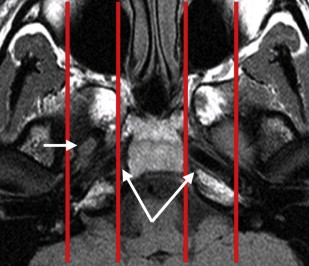
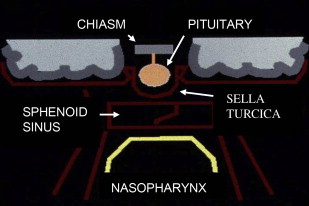
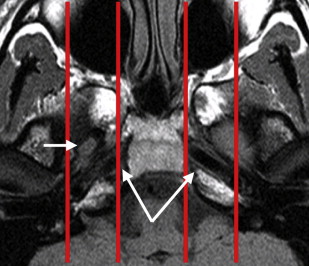
For diagnostic imaging purposes, it is useful to subdivide the CSB further into midline sagittal, off-midline parasagittal, and lateral compartments by drawing vertical lines passing medially to the petroclival fissure and just lateral to the foramen ovale, respectively ( Fig. 2 ). The midline sagittal compartment includes the body of the sphenoid and the portion of the clivus anterior to the spheno-occipital synchondrosis (basisphenoid), contains the sphenoid sinus, and is bordered superiorly by the sella turcica and inferiorly by the roof and posterior wall of the nasopharynx ( Fig. 3 ). It is pierced by the intracranial opening of the orbital apex, giving passage to the optic nerves ( Fig. 4 A ). The parasagittal compartment includes the petroclival synchondrosis, foramen lacerum, and medial aspect of the greater sphenoid wing. It is bordered superiorly and medially by the parasellar region containing the cavernous sinus, superiorly and laterally by the basal temporal lobes, and inferiorly by the parapharyngeal and masticator spaces of the suprahyoid neck. Most of the neurovascular foramina of the CSB lay in this compartment, including the intracranial opening of the superior orbital fissure traversed by cranial nerves III, IV, V1, and VI and the superior ophthalmic vein on their way from the cavernous sinus to the orbit; the foramen rotundum giving passage to V2 on its way from the cavernous sinus to the pterygopalatine fossa; the vidian canal containing the vidian nerve and artery extending from the foramen lacerum to the high pterygopalatine fossa; the foramen ovale traversed by V3 on its way to the masticator space; and the foramen spinosum crossed by the middle meningeal artery. The cavernous sinus, the most important component of the parasagittal CSB, contains, from superior to inferior, cranial nerves III, IV, V1, and V2 enclosed within a dural leaflet of its lateral wall and the cavernous carotid artery and cranial nerve VI within the sinus itself. Finally, the lateral division of the CSB comprises the lateral aspect of the greater sphenoid wing, including the sphenoid triangle, temporal squamosa, and the glenoid cavity of the temporomandibular joint (TMJ).
In addition to location, knowing the major tissue constituents of the CSB can help in establishing further possible differential diagnoses for a skull base lesion. Although some tissues are common to all subdivisions of the CSB, including bone and the pachymeninges investing the intracranial aspect of the skull base, others are site specific. The surgical approach to CSB lesions also varies according to lesion location in each of these compartments (transsphenoidal, transmastoid, temporopteryonal, and frontotemporal approaches).
Imaging approach to pathologic findings
For diagnostic imaging purposes, it is useful to subdivide the CSB further into midline sagittal, off-midline parasagittal, and lateral compartments by drawing vertical lines passing medially to the petroclival fissure and just lateral to the foramen ovale, respectively ( Fig. 2 ). The midline sagittal compartment includes the body of the sphenoid and the portion of the clivus anterior to the spheno-occipital synchondrosis (basisphenoid), contains the sphenoid sinus, and is bordered superiorly by the sella turcica and inferiorly by the roof and posterior wall of the nasopharynx ( Fig. 3 ). It is pierced by the intracranial opening of the orbital apex, giving passage to the optic nerves ( Fig. 4 A ). The parasagittal compartment includes the petroclival synchondrosis, foramen lacerum, and medial aspect of the greater sphenoid wing. It is bordered superiorly and medially by the parasellar region containing the cavernous sinus, superiorly and laterally by the basal temporal lobes, and inferiorly by the parapharyngeal and masticator spaces of the suprahyoid neck. Most of the neurovascular foramina of the CSB lay in this compartment, including the intracranial opening of the superior orbital fissure traversed by cranial nerves III, IV, V1, and VI and the superior ophthalmic vein on their way from the cavernous sinus to the orbit; the foramen rotundum giving passage to V2 on its way from the cavernous sinus to the pterygopalatine fossa; the vidian canal containing the vidian nerve and artery extending from the foramen lacerum to the high pterygopalatine fossa; the foramen ovale traversed by V3 on its way to the masticator space; and the foramen spinosum crossed by the middle meningeal artery. The cavernous sinus, the most important component of the parasagittal CSB, contains, from superior to inferior, cranial nerves III, IV, V1, and V2 enclosed within a dural leaflet of its lateral wall and the cavernous carotid artery and cranial nerve VI within the sinus itself. Finally, the lateral division of the CSB comprises the lateral aspect of the greater sphenoid wing, including the sphenoid triangle, temporal squamosa, and the glenoid cavity of the temporomandibular joint (TMJ).
In addition to location, knowing the major tissue constituents of the CSB can help in establishing further possible differential diagnoses for a skull base lesion. Although some tissues are common to all subdivisions of the CSB, including bone and the pachymeninges investing the intracranial aspect of the skull base, others are site specific. The surgical approach to CSB lesions also varies according to lesion location in each of these compartments (transsphenoidal, transmastoid, temporopteryonal, and frontotemporal approaches).
Imaging modalities and imaging technique
In assessing the CSB, CT and MR imaging have a complementary role and are often used together to establish the presumptive diagnosis and to depict the full extent of a lesion. MR imaging is preferred to assess the soft tissue component and to determine its relations with adjacent structures. It is the modality with the highest accuracy to depict intracranial extent (dural, leptomeningeal, and brain parenchyma invasion), perineural and perivascular spread, and bone marrow involvement. CT remains the technique of choice to define the bony anatomy of the skull base and to depict the thin cortical margins of skull base neurovascular foramina. It is quite specific in the diagnosis of primary bone lesions (neoplastic and non-neoplastic), is more sensitive than MR imaging in the depiction of calcifications, and can provide information regarding the rate of growth and aggressiveness of a lesion by showing its effect on adjacent bone. Whereas permeative and erosive patterns of bone involvement are associated with aggressive rapidly growing lesions, malignant neoplasms, or infectious processes, bone expansion and remodeling with smooth cortical thinning are usually associated with more benign slow-growing processes.
Therefore, in most institutions with multidisciplinary skull base teams, imaging evaluation of skull base lesions includes a full MR imaging study with gadolinium and a high-resolution CT scan using a bone algorithm during the same visit.
The magnetic resonance protocol should include images in the three orthogonal planes using T1 and T2 weighting, short-tau inversion recovery (STIR), and gadolinium-enhanced T1-weighted images with or without fat supression. Gradient echo T2∗ images may be useful to demonstrate the presence of susceptibility artifacts related to the presence of paramagnetic substances, such as calcifications, blood degradation products, or melanin within a lesion. High-resolution highly T2-weighted sequences (constructive interference in the steady state or driven equilibrium radiofrequency reset pulse) can nicely depict the relation between a lesion and the cisternal course of cranial nerves by showing the nerves as dark structures traveling through the high signal intensity of the cerebrospinal fluid (CSF)–filled cisterns. A conventional spin echo or fast spin echo T1-weighted sequence is the single best sequence to depict bone marrow invasion, shown as replacement of the hyperintense fatty marrow by hypointense material. Intravenous administration of gadolinium is mandatory to depict meningeal invasion and perineural spread and usually maximizes tumor contrast against adjacent structures that do not enhance to the same degree. The use of fat suppression, particularly employing techniques based on frequency-selective fat-suppression pulses, is not consensual. Although it can potentially increase the conspicuity of an enhancing lesion against a fat-containing background, failure of fat suppression attributable to the numerous interfaces seen at the CSB (where bone, air-containing sphenoid sinus, soft tissues, and fat lay close together) is often a problem and may lead to false-positive results. The use of STIR, which is not based on frequency-selective pulses, or contrast-enhanced T1-weighted images with appropriate windowing is preferred by some. High-resolution sensitivity encoding (SENSE) parallel imaging and high-field 3-T MR imaging are being increasingly used to depict fine anatomic detail, particularly to study cranial nerves and the walls of the cavernous sinus.
CT studies of the skull base must include images in at least two orthogonal planes with a slice thickness less than 3 mm. Whenever possible, patients should be imaged using a multidetector helical or volumetric scanner, with a single acquisition in the axial plane further reconstructed in different planes as needed without additional radiation to the patient. When CT is obtained as an adjunct to a prior contrast-enhanced MR imaging study, orthogonal images in a high-resolution bone algorithm should suffice. When CT is used as a single examination, a full contrast-enhanced study should be obtained in soft tissue and bone algorithms.
CT and open-field MR imaging scanners can be used for image guidance of fine-needle aspiration cytology or core biopsy to obtain a tissue sample for pathologic diagnosis. Magnetic resonance angiography and CT angiography can be powerful adjuncts in the depiction of vascular malformations and in assessing hypervascular lesions.
18 F-fluorodeoxyglucose positron emission tomography is essentially used in the follow-up of skull base tumors to differentiate post-treatment changes from persistent or recurrent neoplasm. No routine role has been found in the primary evaluation of patients presenting with CSB lesions, however.
Conventional angiography is mainly used for interventional procedures and to assess the circle of Willis when a carotid artery needs to be sacrificed. Preoperative embolization of hypervascular lesions, such as juvenile nasopharyngeal angiofibromas (JNAs), paragangliomas, a few meningiomas, hemangiopericytomas, and hypervascular metastasis, has greatly reduced surgical mortality and morbidity related to bleeding.
Major imaging issues and diagnostic dilemmas: how to report a central skull base imaging study
To issue a useful imaging report, the radiologist should be aware of the main factors that influence treatment options and may have an impact on the surgical approach. To plan treatment, the surgeon needs to know the exact location and extent of a skull base lesion to decide whether it can be excised with acceptable morbidity and to choose the best surgical approach and the best route to obtain a biopsy specimen.
Specific contraindications for surgical resection of skull base lesions vary from one institution to another and often among different surgeons. Usually, they comprise invasion of the lateral or superior walls of the sphenoid sinus, invasion of the cavernous sinus, bilateral optic nerve or optic chiasm involvement, and invasion of the nasopharynx or prevertebral fascia.
On every imaging study of the skull base, the radiologist should always report on the amount of bony skull base involvement; on the presence and extent of intracranial and orbital invasion; and on the relation of the lesion to the cavernous sinus, cranial nerves, and vessels, all of which have important implications on treatment planning and prognosis.
Dural invasion is the single most important prognostic factor in skull base tumors. Whereas an extradural lesion can be resected by means of an inferior ENT approach, a lesion that has transgressed the dura requires a combined craniofacial approach and is associated with a significant decrease in the 5-year survival rate and the specific disease-free interval. Contrast-enhanced MR imaging is the best imaging modality to depict dural invasion. Imaging signs of dural invasion in decreasing order of positive predictive value include contrast enhancement or edema of the brain parenchyma adjacent to tumor, leptomeningeal enhancement, nodular dural enhancement, and linear dural enhancement thicker than 5 mm. Dural enhancement less than 5 mm is the most sensitive but least specific of all imaging signs because it can result from fibrovascular changes and does not necessarily indicate dural invasion.
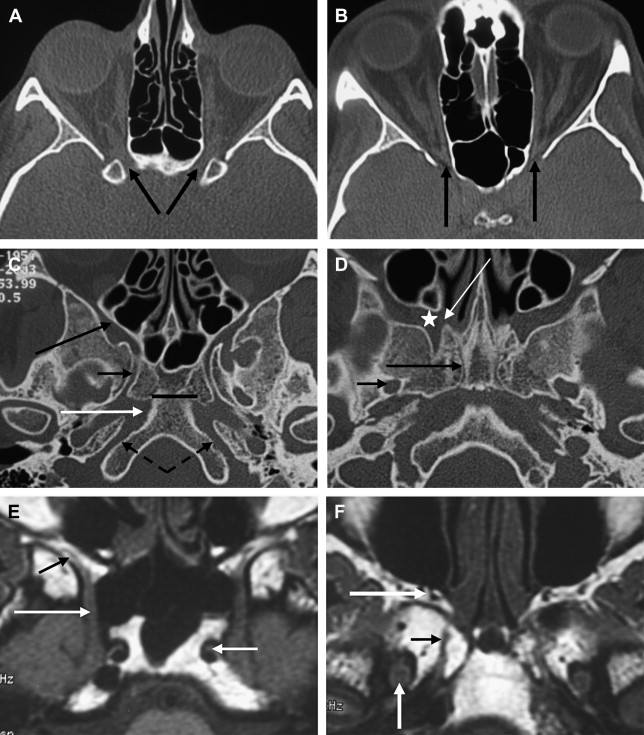
Orbital involvement is another important issue in surgical planning. The most common site of orbital invasion by CSB lesions is by way of the orbital fissures or orbital apex ( Fig. 5 D ). Loss of fat and abnormal enhancement within these neural foramina indicate invasion. Because of the close relation between the periorbita and the dural sheath of the optic nerve, involvement of the orbital apex by a skull base lesion usually requires orbital exenteration with sacrifice of the optic nerve.
Invasion of the cavernous sinus usually precludes complete resection of a lesion. Imaging signs of cavernous sinus invasion include compression, encasement, stenosis, or irregularity of the cavernous carotid artery; loss of contrast enhancement of the cavernous sinus, which is best depicted on a dynamic coronal MR imaging study; and bulging of the lateral sinus wall, which is concave under normal conditions (see Fig. 5 E, F). When subtle, cavernous sinus invasion may be hard to depict, even using optimal technique. Because the dural leaflets are not clearly visible, it is often difficult to differentiate a bulge of the sinus walls because of compression by an extrinsic lesion from actual dural transgression. The use of 3-T MR imaging scanners seems promising in solving this issue. It should also be kept in mind that the cavernous sinus may be affected by interdural lesions laying within the dural leaflets of the lateral wall or by true intracavernous lesions. Whereas the former tend to displace the cavernous carotid artery without encasement or stenosis, the latter tend to encase and narrow its lumen.
Pathologic findings
Most patients with CSB problems present with headache, cranial nerve deficits, proptosis, Eustachian tube dysfunction, or other symptoms related to the nasopharyngeal airway. Because clinical examination of the CSB is limited, surgeons often have to rely on imaging studies to reach a diagnosis and plan subsequent treatment.
The following description of CSB lesions is based on a radiologist’s friendly approach and on the frequency and propensity for certain disease processes to occur at specific sites. Lesions that are not site specific, originating from the bony skull base, such as fibro-osseous conditions and primary and secondary bone tumors, are discussed at the end of this article.
Midline Sagittal Central Skull Base Lesions
Midline sagittal CSB lesions comprise lesions arising from the sphenoid body, sphenoid sinus, and clivus; from the sella turcica immediately above; and from the nasopharynx immediately below (see Fig. 3 ). Whereas lesions arising within the sella turcica tend to displace the sellar floor inferiorly, intrinsic lesions of the clivus and those arising from the nasopharynx tend to push the sellar floor and pituitary gland superiorly.
Lesions of the sphenoid sinus can be inflammatory and neoplastic. Chronic obliteration of sinus drainage may lead to mucocele formation. Sphenoid mucoceles, as is the case with those seen elsewhere, present as expansile lesions thinning and remodeling the sinus walls and eventually leading to cortical bone dehiscence. This tumor, made of retained secretions, may show variable density and signal intensity on imaging studies depending on the degree of hydration, protein concentration, and presence of calcification or fungal colonization (mycetoma) and does not enhance after intravenous administration of contrast material, except for a thin peripheral rim corresponding to the sinus mucosa ( Fig. 6 ). The imaging appearance is that of a benign slow-growing lesion, and the diagnosis is usually straightforward in the absence of complications.
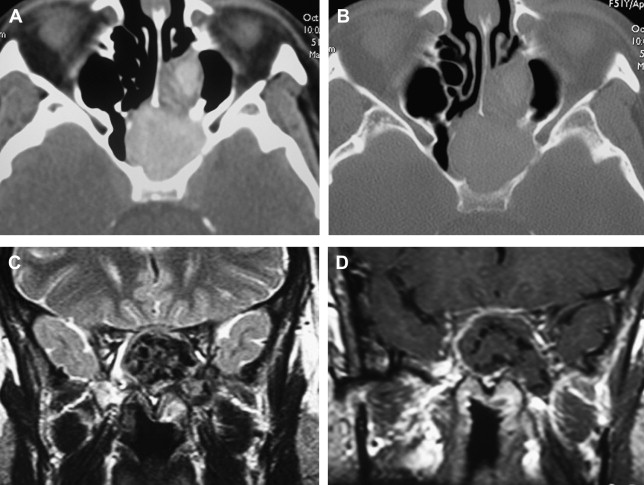
Primary neoplasms of the sphenoid sinus are quite rare, because the sphenoid is most often secondarily invaded by tumors originating elsewhere. Tumor histologic findings are similar to those seen in other paranasal sinuses, with squamous cell carcinoma being the most common.
Clivus lesions include hematogenous bone metastasis, plasmacytoma or multiple myeloma, primary bone tumors, chordoma, and chondrosarcoma.
Clival chordoma is a benign locally invasive neoplasm originating from embryonic remnants of notochord that become entrapped within the basisphenoid, accounting for its typical midline sagittal location. Seldom can it be seen in the nasopharynx or in the intracranial compartment. Chordomas present as expansile lytic lesions, often with an aggressive pattern of bone destruction. MR imaging can nicely depict the replacement of the fatty marrow of the clivus by a hypointense tissue mass on plain sagittal T1-weighted images. Density and signal intensity are variable because of the presence of hemorrhage, cystic or myxoid components, calcification, and fragments of trabecular bone that become engulfed within the tumor mass. After contrast, a lobulated pattern of enhancement is usually described ( Fig. 7 ).