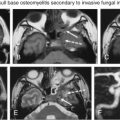
Abstract
Facial nerve dysfunction can occur from a variety of causes, including congenital, inflammatory, traumatic, vascular, and neoplastic processes. Imaging plays an important role in the evaluation of facial nerve disorders. Knowledge of the complex anatomy of the facial nerve is essential to localize the site of pathology. This article reviews the anatomy of the facial nerve and the current clinical evaluation and imaging strategies.
Keywords
Computed tomography, Cranial nerve, Facial nerve, Magnetic resonance imaging
Anatomy
The facial nerve comprises motor, sensory, and parasympathetic fibers. The dominant motor component comprises 70% of the total axons. The sensory component makes up much of the remaining portion of the nerve and includes the nervus intermedius (nerve of Wrisberg). The motor division supplies somatic motor fibers to the muscles of the face, scalp, and auricle; the buccinators; the platysma; the stapedius; the stylohyoideus; and the posterior belly of the digastric. The motor division also contains sympathetic motor fibers, which constitute the vasodilator nerves of the submandibular and sublingual glands, conveyed through the chorda tympani nerve. The nerve of Wrisberg contains lacrimal secretomotor parasympathetic fibers and sensory fibers carrying input from the external auditory canal, the nasopharynx, and palate and taste sensation from the anterior two-thirds of the tongue and oral cavity. The facial nerve also has many clinically relevant communications with other cranial and cervical spinal nerves.
Supranuclear Control
The supranuclear contribution from the motor cortex ( Fig. 9.1 ) originates from pyramidal neurons in the lower third of the precentral gyrus. The cortical projection fibers pass within the posterior genu of the internal capsule and descend in the pontine pyramidal tracts. The fibers for the lower facial muscles completely decussate to the contralateral facial nucleus. However, some cortical projection fibers for the upper face project to the ipsilateral as well as the contralateral facial nuclei. This explains why supranuclear lesions spare the motor functions of the upper face.
Motor Component
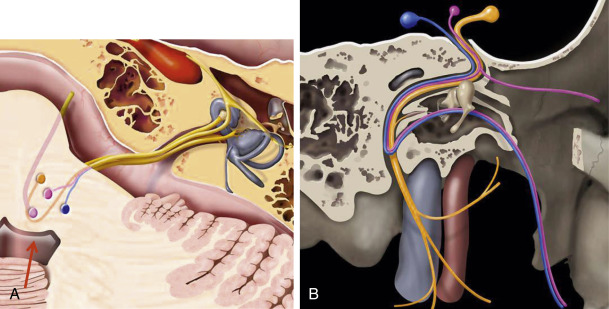
The motor nucleus of the facial nerve is located within the pontine tegmentum, just superior to the nucleus ambiguus, posterior to the superior olivary nucleus, and medial to the spinal trigeminal nucleus. The fibers course posteromedially and loop (the internal genu) around the medial aspect of the abducens nucleus to form the facial colliculus, a shallow bulge in the floor of the fourth ventricle, easily identified on axial MR images. This close anatomic relationship explains how both the facial and abducens nerves may be simultaneously affected by disease processes. The motor fibers exit the lateral border of the pons in the cerebellopontine angle cistern, where they lie medial to cranial nerve (CN) VIII ( Fig. 9.2 ).

Sensory, Special Sensory, and Parasympathetic
The nerve of Wrisberg exits the pons between the motor root and CN VIII and courses parallel to the motor root, to the internal auditory canal. It also carries somatosensory fibers (from the trigeminal nucleus, having received them from the posterior auricular nerve) and special sensory taste fibers, received from the chorda tympani from the anterior tongue and oral cavity, to the nucleus tractus solitarius in the medulla.
The parasympathetic secretomotor component comprises fibers to the lacrimal and submandibular glands that originate in the superior salivatory nucleus. At the geniculate ganglion, the fibers to the lacrimal gland do not synapse but leave via the greater superficial petrosal nerve. They then join the deep petrosal nerve (comprising sympathetic nerve fibers) to form the vidian nerve (of the pterygoid canal) and synapse in the pterygopalatine ganglion. Postganglionic fibers reach the lacrimal gland via the zygomatic branch of the maxillary nerve (V2), which communicates with the lacrimal nerve (branch of the V1).
The preganglionic fibers for the submandibular and sublingual glands continue along the facial nerve, passing through the geniculate ganglion, and exit at the mastoid segment within the chorda tympani. The chorda tympani travels medial to the handle of malleus in the tympanic cavity and enters the infratemporal fossa through the petrotympanic/glaserian fissure, where it joins the lingual nerve (CN V3). The fibers synapse within the submandibular ganglion, innervating the submandibular and sublingual salivary glands.
Peripheral Course
The facial nerve may be conveniently divided into cisternal, intracanalicular, labyrinthine, tympanic, mastoid, and extracranial segments ( Fig. 9.3 ). The facial nerve exits from the pontomedullary sulcus at the root exit point. The nerve remains adherent to the undersurface of the pons for 8–10 mm, through the attached segment. The nerve then detaches from the pons at the root detachment point. The transition zone extends for approximately 2 mm, followed by the cisternal segment, which extends anterolaterally to the porus acusticus. The cisternal segment is typically 24 mm in length.

The nerve of Wrisberg exits between the motor root and the vestibulocochlear nerve. The nerve joins the motor root as it exits the brainstem or at the porus acusticus and becomes a common trunk, the nervi facialis. The intracanalicular segment travels within the anterior and superior quadrants of the internal auditory canal (IAC) for approximately 8 mm.
Within the lateral IAC, the nerve is separated from the cochlear nerve inferiorly by a horizontal partition of dura and bone, the transverse or falciform crest. A vertical dural and osseous crest known as Bill bar separates the facial nerve from the superior vestibular nerve, which lies posteriorly ( Fig. 9.3 ).
The facial/fallopian canal consists of three segments: labyrinthine, tympanic, and mastoid, extending from Bill bar to the stylomastoid foramen ( Fig. 9.3 ). The labyrinthine segment is the narrowest and shortest segment within the facial canal, 3–5 mm in length. It extends from the anterosuperior fundus to the geniculate ganglion. It passes between the ampulla of the superior semicircular canal and the cochlea to travel forward and downward. Exiting anteriorly from the geniculate ganglion is the first branch of CN VII, the greater superficial petrosal nerve (GSPN). At the geniculate ganglion, the nerve makes a sharp 75 degree turn posteriorly (anterior or first genu). This marks the beginning of the tympanic segment. The tympanic segment ( Fig. 9.3 ) is 10 mm in length. The nerve traverses the medial wall of the tympanic cavity superolateral to the oval window and inferior to the lateral semicircular canal. It then enters the facial recess, medial to the pyramidal eminence, and then turns inferiorly 95–125 degrees (posterior or second genu) to become the mastoid segment. The mastoid segment courses posteromedial to the external auditory canal and lateral to the inferior aspect of the tympanic annulus, extending for 13 mm in adults. The mastoid segment gives rise to the nerve to the stapedius and the chorda tympani.
The extracranial facial nerve exits the temporal bone at the stylomastoid foramen, situated between the styloid process anteriorly and the mastoid process posteriorly. The nerve immediately enters the substance of the parotid gland, after which it divides into two terminal branches: the upper temporofacial branch and the lower cervicofacial branch. In the parotid gland, these nerves further divide into temporal, zygomatic, buccal, marginal mandibular, and cervical branches.
The peripheral nerve is composed of three layers, the endoneurium, the perineurium, and the epineurium. The intracranial and intracanalicular segments of the nerve lack the perineurium and epineurium, making them more susceptible to injury.
Vascular Supply of the Facial Nerve
The cortical motor area of the face is supplied by the middle cerebral artery. The facial nucleus within the pons is supplied primarily by the anterior inferior cerebellar artery (AICA), a branch off the basilar artery. The AICA enters the IAC with the facial nerve and gives rise to the labyrinthine (internal auditory) artery and supplies the cisternal, intracanalicular, and labyrinthine segments. The tympanic and mastoid segments are fed from the stylomastoid artery, typically arising from the posterior auricular artery. The extracranial facial nerve is supplied by posterior auricular, occipital, and superficial temporal arteries.
Venous drainage parallels the arterial blood supply. Within the IAC, the veins drain into branches of the internal auditory vein. At and distal to the geniculate ganglion, the nerve is surrounded by a tough connective tissue sheath, which is contiguous with the facial canal periosteum and the epineurium. Within this sheath lies a venous network, which drains into veins accompanying the petrosal and stylomastoid arteries. This accounts for normal enhancement of the geniculate ganglion and distal nerve segments on postcontrast MRI ( Fig. 9.4 ).

Clinical and Imaging Evaluation
Facial paralysis may occur in a variety of acute and chronic clinical settings. A variety of infectious, autoimmune, granulomatous, neoplastic, and vascular disease can present with facial palsy. The first step in clinical evaluation is the determination of whether the facial palsy is consequent to an upper or lower motor neuron process. Clinical evaluation of facial weakness centers on the determination of whether the weakness is caused by a central (supranuclear) or a peripheral (nuclear, lower motor neuron) lesion. A useful clinical tenet is that a central lesion will spare the forehead and brow, whereas a peripheral lesion will result in complete facial weakness in addition to loss of emotional or involuntary facial motion. These presentations result from bilateral supranuclear innervation to the upper facial musculature. The clinician may also be able to localize the lesion to a segment of the nerve based on whether lacrimation is impaired (implying a lesion proximal to the GSPN-suprageniculate), hyperacusis is present (suprastapedia), or if taste and salivation are affected (suprachordal). The severity of facial paralysis is typically graded using the House-Brackmann Facial Nerve Grading System ( Table 9.1 ). Central causes are best investigated with MRI, whereas peripheral causes may require a combination of MRI and CT. Often, disease processes may affect long segments of the nerve and such precise localization is not possible. The most commonly encountered cause of lower motor neuron facial paralysis is Bell palsy, discussed in detail later.
| Grade/Degree of Dysfunction | Clinical Findings |
|---|---|
| I. Normal | Normal function |
| II. Mild | Slight weakness noticeable on close inspection Normal symmetry and tone at rest Slight mouth asymmetry with motion |
| III. Moderate | Obvious, but not disfiguring facial asymmetry Moderate synkinesis, hemifacial spasm Normal symmetry and tone at rest Slight forehead and mouth weakness with motion |
| IV. Moderate to severe | Obvious weakness and/or disfiguring asymmetry No forehead motion, incomplete eye closure, asymmetric mouth motion with maximum effort |
| V. Severe | Only barely perceptible motion Asymmetry at rest No forehead movement, only slight mouth movement, incomplete eye closure |
| VI. Complete paralysis | No facial movement |
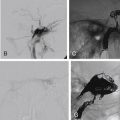
A typical MRI protocol for evaluating the facial nerve and temporal bone should comprise diffusion-weighted images (DWIs); axial and coronal pre- and postcontrast thin-section (3-mm) T1-weighted images, the latter with fat suppression; and a heavily T2-weighted volumetric sequence. In the evaluation of hemifacial spasm (HFS), MR angiography is useful in the identification of a compressive vascular loop. The intraparotid segment of the nerve is not usually well demonstrated on routine MRI but may be visualized using a steady state gradient echo sequence, such as three-dimensional (3D)-reversed fast imaging with steady-state precession (PSIF)-DWI (Siemens, Erlangen, Germany) or a 3D FIESTA (fast imaging employing steady state precession; GE, Milwaukee, USA) sequence or its equivalent. MR Diffusion Tensor Imaging (DTI) tractography has been used to demonstrate the position of the facial nerve in relation to CerebelloPontine Angle (CPA) tumors.
The normal facial nerve on contrast-enhanced MRI exhibits enhancement in its tympanic segment and beyond because of the presence of a circumneural venous plexus. It is important to note that on both unenhanced and contrast-enhanced 3D inversion recovery-prepared fast spoiled gradient-echo, the normal nerve may appear increased in signal intensity ( Fig. 9.4 ).
CT is best performed with a multidetector CT scanner, with images acquired axially parallel to the hard palate, with an edge-enhancing algorithm. Images are viewed at a wide window level and width and are displayed with a small field of view. The axial images are reconstructed typically in coronal and sagittal planes (although they may be reconstructed in any plane, given that the voxels are isotropic).
Congenital Anomalies
Congenital malformations of the facial nerve may occur in isolation or in association with more complex abnormalities of the brain and temporal bones. They may be asymptomatic or present at birth with profound facial paralysis. Bifurcations and trifurcations of the nerve may occur as isolated phenomena, most commonly distal to the geniculate ganglion, in the tympanic segment, although rare labyrinthine and mastoid segment bifurcations have also been reported.
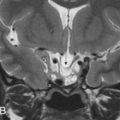
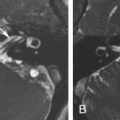
Incomplete closure of the fallopian canal caused by a malformation of Reichert cartilage results in dehiscence of the canal, most commonly at the level of the oval window. A pure dehiscence without prolapse of the nerve cannot be reliably diagnosed using CT because the wall of the normal facial nerve canal is extremely thin. The nerve can prolapse into the middle ear cleft and lie directly over the oval window and may be at risk for inadvertent injury at surgery. Although this is typically an incidental finding, the occasional patient may present with conductive hearing loss consequent to the stapedial crura being attached to the nerve. On coronal CT images, the prolapsed nerve is readily identified as a smoothly convex soft tissue structure inferior to the lateral semicircular canal, overlying the oval window and stapes, contiguous with the facial nerve canal ( Fig. 9.5 ). Facial nerve prolapse is also a feature of oval window atresia and the CHARGE syndrome (colobomas, heart defects, choanal atresia, mental retardation, and genitourinary and ear anomalies) ( Fig. 9.5 ).

The course of the facial nerve is frequently altered in congenital aural atresia and is an important variable that determines surgical planning. Typically, the angle at the first genu is excessively obtuse, the tympanic segment is displaced inferiorly to lie over the oval window, and the second genu and mastoid segments are displaced anteriorly. The nerve may therefore exit the temporal bone more anteriorly, at the level of the round window, increasing the risk of injury at surgery when the auricle is elevated. The course of the nerve is also altered with malformations of the oval window and inner ear structures ( Fig. 9.5 ).
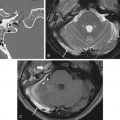
The facial nerve and its nucleus may be congenitally absent, as has been noted in Mobius (where the abducens nerve is also abnormal), DiGeorge, Poland, Goldenhar, and trisomy 13 and 18 syndromes, among others. Absence or hypoplasia of the nerve is best demonstrated on high-resolution 3D T2-weighted sequences. On sagittal reformatted images derived from such a sequence, the nerve will be absent from its usual position in the anterosuperior quadrant of the IAC ( Fig. 9.6 ). The facial nerve also may demonstrate an anomalous course when the vestibulocochlear nerve is absent. Aplasia, aberrant origin, and course of the facial nerve may be a feature of pontine tegmental cap dysplasia, a rare hindbrain malformation. In such patients, the facial and vestibular nerves course in two separate anomalous bony canals (duplicated IAC) ( Fig. 9.7 ).