Interpreting CT or MR imaging examinations performed on patients with a history of middle ear, mastoid, or neurotologic surgery can be challenging. This is greatly simplified by knowing the surgical procedures used and the expected postoperative appearance. These are reviewed and illustrated in this article. Knowing the normal postoperative appearance is the key to recognizing signs of recurrent disease.
Interpreting CT or MR imaging examinations performed on patients with a history of middle ear (ME), mastoid, or neurotologic surgery can be challenging. This is greatly simplified by knowing the surgical procedures used and the expected postoperative appearance. These are reviewed and illustrated. Knowing the normal postoperative appearance is the key to recognizing signs of recurrent disease.
Mastoid and middle ear
The goal of surgery in the ME and mastoid is the elimination of disease and, if possible, the preservation or restoration of normal function. The following approaches and procedures may be performed singularly or in combination.
Transcanal Approach
This approach is used for disease processes that can be adequately visualized in the external auditory canal (EAC) with the entire surgery performed through the EAC. Areas accessible include the EAC; central tympanic membrane (TM); the central ME; ossicles (to include the stapes); tympanic sinus; and portions of the facial recess and hypotympanum. Visualization of the anterior portions of the EAC near the TM and protympanum is limited. It is the preferred approach for EAC stenosis, exostosis, osteomas, myringitis, repair of central perforations, placement of pressure equalization tubes, and so forth. There may be little evidence of postoperative change in the EAC. A widened appearance to the EAC may be evident from drilling of bone at the level of the isthmus. Advantages include limited surgical dissection so postoperative pain is diminished. As a result, patients normally return to their presurgical functional status within 1 to 2 days.
Postauricular Approach
An incision is made posterior to the ear, which is reflected anteriorly. The mastoid cortex and subjacent air cells are drilled away providing exposure to the ME and mastoid. It is the primary approach for many neurotologic procedures, such as the translabyrinthine, retrosigmoid, or retrolabyrinthine surgery. It provides the best exposure to the EAC and facilitates tympanoplasty when the transcanal approach cannot give the necessary visualization. It does require the use of a mastoid dressing that can be removed the next day. The postoperative recovery period is 4 to 5 days longer than that of the transcanal approach.
Canaloplasty
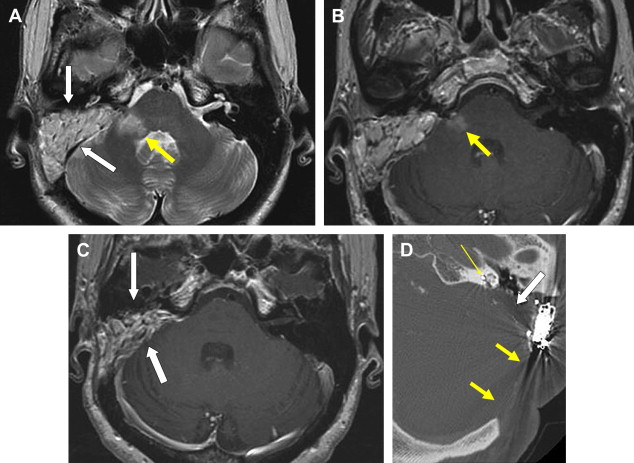
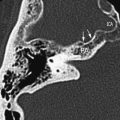
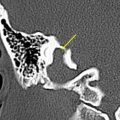
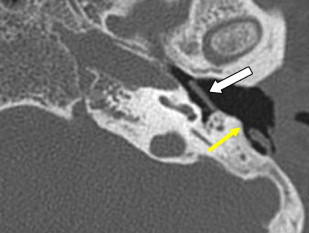
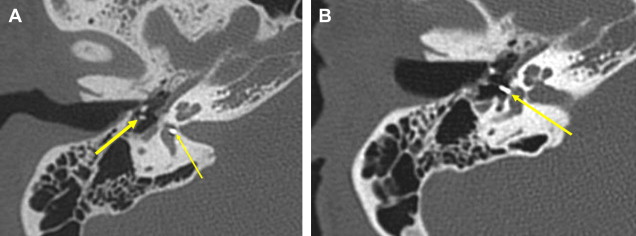
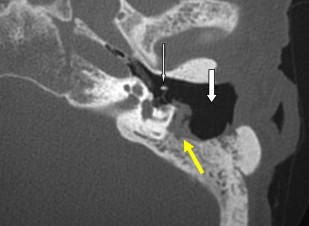
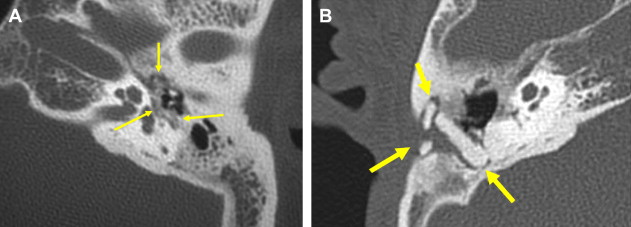
This is a procedure enlarging the EAC facilitating immediate or future surgery by the transcanal approach. Postoperative change on imaging may be minimal in cases of EAC stenoses or could be extensive in patients with EAC atresia. The most common finding on CT is a “box-like” appearance to the EAC from bone drilling at the isthmus level and loss of bone anteriorly deep in the EAC secondary to “blue lining” giving the appearance of an EAC–temporomandibular joint “fistula” ( Fig. 1 ).
Myringoplasty
Myringoplasty, also known as “type 1 tympanoplasty,” refers to surgery performed on the eardrum. The aim of myringoplasty is to restore the normal functions of the TM. The procedure may be simple, such as patching small perforations, or more complex to include lasering of chronic myringitis, removal of tympanosclerotic plaques, or reconstructing the entire TM with fascial grafts. The TM can be quite variable in appearance on CT, appearing so thin as to be barely perceptible or thickened focally and diffusely ( Figs. 1 and 2 ). Blunting of the anterior angle is common, this “acute” angle formed by the TM and the adjacent anterior wall of the EAC. The posterior angle is more obtuse and less commonly blunted. The presence of a pressure equalization tube is common and should be noted.
Tympanoplasty
Although tympanoplasty and myringoplasty appear synonymous, the term “tympanoplasty” includes surgery involving the TM, ossicles, and associated ME disease.
Ossiculoplasty
Surgery is performed to reconstruct the continuity of the ossicular connection between the TM and graft and the oval window. This reconstruction may involve repositioning of ossicles, usually the incus. The incus may be reshaped for proper fitting extending from the drum or the malleus to the stapes superstructure or footplate. Various forms of ossicular prosthesis are available extending from the TM and malleus handle to the stapes superstructure (partial ossicular prosthesis) or from eardrum and malleus handle to the stapes footplate (total ossicular prosthesis). The materials used are numerous and may be used in combination to prostheses made from calcified particles or hydroxyapatite; metal (eg, titanium); or plastic (plastipore). Stacked cartilage and bone cement may also be used. Specific prostheses are not discussed here because they are numerous. It is best to become familiar with the prosthesis used locally by one’s surgeons.
Stapedectomy
Stapedectomy specifically refers to complete removal of the stapes and footplate. There is significant variation, however, in “stapes surgery” in that a portion of the stapes superstructure may remain along with the footplate. The arch only may be removed and the footplate mobilized. A stapedotomy refers to a hole made in an immobile footplate through which prosthesis is placed.
There are five variations of tympanoplasty: (1) myringoplasty, ossicles intact; (2) TM perforation with diseased malleus or incus, most commonly the long process of the incus; (3) intact mobile stapes with destruction of malleus and incus; (4) destruction of ossicles (including stapes arch) with mobile footplate; and (5) destruction of ossicles as in type 4 with fixed footplate.
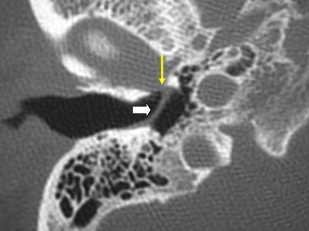
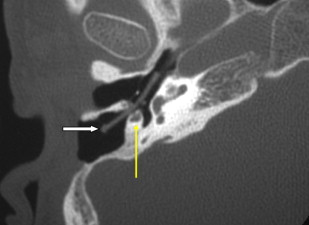
There are two important points regarding tympanoplasty-ossiculoplasty. First, when reading CT examinations, look for continuity of the ossicular chain–prosthesis from the TM and graft to the stapes or footplate ( Figs. 3 and 4 ). Off-axis reformatted images are often valuable for this purpose. The ossicular reconstructions used are quite variable and not always “straight line” or centered on the footplate ( Fig. 5 ). The prosthesis may extend just deep to the footplate but should not extend deep into the vestibule ( Fig. 6 ). It is important to remember that the apparent penetration below the footplate estimated by CT may be greater than the actual penetration anatomically. Secondly, audiometry trumps imaging. That is to say, if there is significant conductive hearing loss, even with a “satisfactory” imaging appearance the patient likely needs revision surgery. Displacement of the prosthesis by only 0.1 to 0.2 mm is sufficient to cause conductive hearing loss and is difficult to detect by CT, particularly if there is artifact from metal.
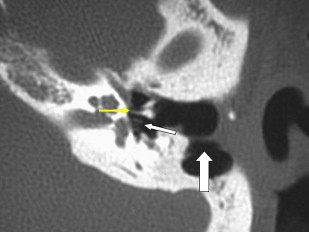
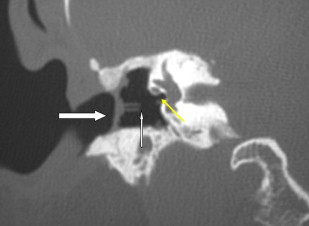
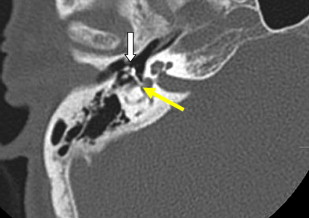
Mastoidectomy
The term “mastoidectomy” is a misnomer because the mastoid is not removed in its entirety. The term refers to removal of the mastoid cortex and portions of the mastoid that are diseased to include aircells, the mastoid tip, the sinodural angle, and tegmen. Anterior and superiorly, the surgery may extend to the epitympanum and the anterior attic region with the geniculate ganglion the anterior most landmark. The size of the mastoidectomy defect is quite variable depending on the degree of mastoid pneumatization, extent of disease, and procedures performed. The smallest defects are seen in patients with sclerotic mastoids and no mastoid disease, the surgery performed for placement of an endolymphatic shunt. Large defects are seen in patients with well-pneumatized mastoids with extensive disease requiring complete air cell exenteration.
Radiologists should make note of a very sclerotic mastoid, especially those with almost no aeration of the mastoid antrum. In these patients the transmastoid approach can be dangerous because it is difficult to delineate the lateral semicircular canal and second genu of the facial nerve, both of which may be injured at the time of surgery. On postoperative scans in these patients it is common to see small surgical defects in bone over the inferior temporal region above the tegmen, created during attempts to enter the mastoid.
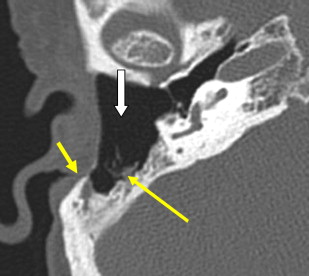
The posterior wall of the EAC may be left intact or resected. When intact, this is commonly referred to as a “closed” mastoidectomy or mastoidectomy with intact posterior canal wall ( Fig. 7 ). If the posterior canal wall is removed, this is referred to as mastoidectomy with takedown of the posterior canal wall or “open” mastoidectomy (see Fig 3 ; Fig. 8 ). On rare occasions, the posterior canal wall may be removed, allowing wider access to the ME and mastoid, then replaced or reconstructed. The choice of performing an intact canal wall versus canal wall down mastoidectomy is dependent on whether future mastoid surgery is anticipated because these procedures are usually performed in patients with cholesteama. With the canal wall down mastoidectomy and meatoplasty, additional surgery is required only in 10% of patients. With the intact canal wall mastoidectomy, a “second look” mastoidectomy is required because the recurrence of cholesteatoma is approximately 20% to 30%, even if the surgeon believes all cholesteatoma was removed grossly during the initial operation. A common site for recurrent cholesteatoma is around the oval window because complete surgical removal of cholesteatoma around the facial nerve or that adherent to the mobile stapes footplate can result in significant facial nerve paralysis or significant sensorineural hearing loss, tinnitus, and dizziness with vertigo.
Facial Recess Approach
This is performed with an intact canal wall mastoidectomy with an opening made just medial to the chorda tympanic nerve and just lateral to the mastoid segment of the facial nerve. This is used for cochlear implant surgery and also as a means to removal of disease in the facial recess (see Fig. 7 ).
Atticotomy
An atticotomy refers to removal of the lateral wall of the epitympanum. This is not typically described separately if the patient is posttympanoplasty or mastoidectomy because the lateral wall may be removed as part of the mastoid procedure. On occasion, however, atticotomy is performed alone and this should be noted. The mastoid cortex and posterior canal wall appear normal. An atticotomy is best visualized on coronal images.
Postoperative Imaging
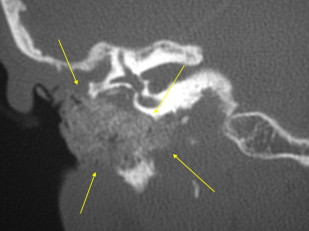
Patients are not typically imaged acutely unless complications are suspected. Immediately after surgery, it is normal to see soft tissue swelling, absorbable gelatin sponge, mastoid cell opacification, and fluid levels ( Fig. 9 ). For intracranial complications, such as stroke, dural sinus thrombosis, abscess, or cerebritis, MR imaging, MR angiography, and MR venography are preferred. CT has a limited role but may be useful if there is clinical concern for cerebrospinal fluid (CSF) leak, facial nerve injury, or labyrinthine fistula.
In the more typical case, patients are imaged months or years after surgery because of recurrent hearing loss, inflammatory disease, or cholesteatoma. When imaging is normal, there is good aeration of the ME and mastoid without fluid. The EAC has normal margins without soft tissue thickening. The TM appears thin and intact. The ossicles or prostheses are intact and in continuity. The otic capsule appears normal without erosion, fistula, or pneumolabyrinth.
Postoperative CT imaging after mastoidectomy is very challenging with regard to differentiating acute disease versus chronic, nonactive disease. Mild soft tissue thickening with otherwise good aeration is commonly seen and likely represents scar or granulation tissue. More extensive thickening, especially when accompanied by fluid or complete opacification of the ME and mastoid, is more likely to represent active disease. It is common to see soft tissue opacification of residual mastoid air cells and around the margins of the mastoid defect, especially if the margins are irregular with “overhanging” edges (see Fig. 8 ; Fig. 10 ). Patients with eustachian tube dysfunction often have retained fluid. Because of this, it is important to recognize that “mastoiditis” is a clinical diagnosis supported by imaging findings.
Recurrent cholesteatoma may be difficult to diagnose with certainty by CT, especially so when it is surrounded by inflammatory disease or fluid. It is important to know that after surgery, cholesteatoma may recur in the EAC, TM, ME, or mastoid. Progressive bone erosion should always raise concern but this requires a baseline postoperative examination (rarely indicated) to differentiate from preoperative erosion or operative drilling. A focal round soft tissue mass is suspicious but uncommon. On occasion, a mass has irregular air-filled interstices from shedding of keratin debris. Rarely, the cholesteatoma contents are shed or removed with only the peripheral matrix remaining, a so-called “thin wall” cholesteatoma.
If a mass is noted on the postoperative CT, differential considerations other than cholesteatoma include encephalocele-meningocele and cholesterol granuloma (see Fig. 10 ; Fig. 11 ). It is important to inspect the tegmen to be sure it is intact ( Fig. 12 ). MR imaging without and with contrast is useful in differentiating encephalocele, inflammatory disease, cholesteatoma, and cholesterol granuloma but is infrequently obtained because it seldom alters clinical management.
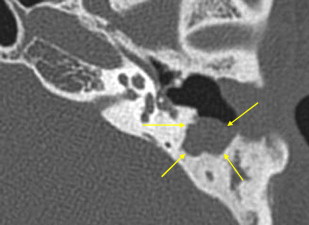
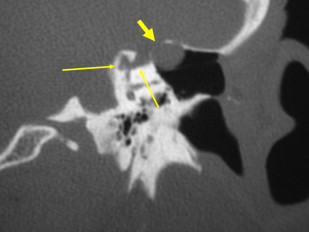
Hazy ill-defined new bone formation is occasionally seen about the margins of the mastoid and ME. When seen, this should suggest active inflammation ( Fig. 13 ).
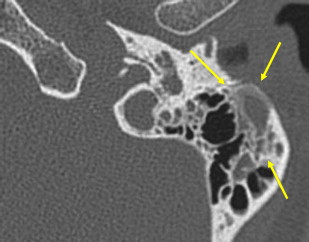
Tympanosclerosis refers to fibrous tissue, calcification, or new bone formation in response to chronic otitis media ( Fig. 14 ). It is important to note the presence and extent of tympanosclerosis. It is a cause of conductive hearing loss poorly visualized by the surgeon. Tympanosclerotic fixation of the malleus and incus occurs in the lateral epitympanum or involves the stapes footplate. The scarring obliterates normal ME landmarks that the surgeon relies on for orientation. This increases morbidity and results in longer operative times.
Internal auditory canal and cerebellopontine angle
Acoustic schwannomas (AS) are the most commonly encountered extra-axial internal auditory canal (IAC)–cerebellopontine angle (CPA) tumors followed in frequency by meningiomas. These lesions along with epidermoids account for over 90% of CPA tumors. Over 60 other tumors account for the other 10%. Tumors may present as a purely intracanalicular lesion (AS, meningioma, facial neuroma, hemangioma, lipoma, and so forth); within the CPA; or in both regions.
There are three options available to the clinician once a tumor is diagnosed: (1) observation, (2) radiotherapy, or (3) surgery. This article does not discuss radiotherapy or imaging algorithms for observation. There are three standard approaches to the IAC-CPA region with variations described in the literature. The approach used depends more on the expertise and training of the surgeon rather than the size or location of the lesion.
Retrosigmoid Approach
The retrosigmoid approach is a commonly used technique used for removal of tumors in the IAC and CPA. It is a team approach. The neurosurgeon performs the craniotomy with cerebellar retraction and removal of the intracranial portion of the tumor with the neurotologist performing any required drilling around the IAC with subsequent tumor removal in the IAC. Advantages are that tumors of any size can be removed. There is excellent exposure to the CPA, lower cranial nerves, brainstem, and cerebellum. CN7 and CN8 are easily identified. After drilling of the IAC and removal of the tumor in the temporal bone–IAC, the most difficult part of the surgery is the removal of the tumor around the porous of the IAC because the facial nerve is often splayed and adherent to the capsule of the tumor.
Monitoring of the facial nerve (CN7) significantly reduces the risk of facial paresis and paralysis. The use of intraoperative auditory nerve monitoring also reduces the risk of losing the hearing in small acoustic tumors, usually those confined in the IAC.
A weakness of this approach is that the lateral end of the IAC cannot be exposed without potentially damaging hearing. The lateral 2 to 3 mm of the IAC cannot be drilled out without drilling into the posterior semicircular canal or into the vestibule, both of which could result in significant sensorineural hearing loss. This is not a concern if there is profound hearing loss preoperatively. If hearing is to be spared, then the lateral 2 to 3 mm of the IAC is not drilled and tumor is left in this area. If the labyrinth is violated, preservation of the lateral semicircular canal is most critical. Careful removal of the superior and posterior semicircular canals can be performed without significant loss of hearing over 80% of the time (transcrusal approach).
Translabyrinthine Approach
This approach is used if there is no useful hearing on the side of the tumor and there is no concern for hearing preservation. Following an extended mastoidectomy the labyrinth is drilled away with removal of all three of the semicircular canals and opening into the vestibule. The facial nerve can be followed directly from the second genu in the mastoid to the geniculate and points the way into the IAC. This approach was developed by Drs. William House and William Hitselberger for identification of the facial nerve. When developed, there was no intraoperative facial nerve monitoring available. The bone over the sigmoid sinus is removed or islanded to allow placement of a retractor and better exposure in the CPA. Subsequently, bone of the IAC is removed with tumor in the IAC and CPA exposed and resected. The size of the defect depends on the exposure required. The cochlea is commonly spared.
This approach can be used for tumors of all sizes, exposure facilitated by extensive drilling of bone lateral to the tumor. Wide access nearly to the midline can be achieved, extending anteriorly to the carotid and posteriorly to the sinodural plate. After tumor removal, the medial defect is covered with fascia and then filled laterally with fat. There is complete hearing loss after surgery. Advantages include early identification of CN7 lateral to the tumor (in the case of AS) with low incidence of tumor recurrence. The incidence of headache is low with the incidence of CSF leak similar to that of the posterior fossa approach (about 5%). Disadvantages include lack of visualization of the brainstem until the tumor is resected and limited familiarity of the approach to neurosurgeons should complications arise.
Middle Cranial Fossa Approach
Following a subtemporal craniotomy, the dura along the floor of the middle cranial fossa is elevated along with the temporal lobe extradurally. The dura covering the middle cranial fossa floor is elevated medially to the petrous ridge, usually from a posterior to anterior manner with the superficial petrosal sinus as the medial landmark.
The anterior limit is the middle meningeal artery. Care is taken during dural elevation from posterior to anterior looking for dehiscence of the greater superficial petrosal nerve (40%–50%) or the superior semicircular canal (5% or more). This could result in facial nerve injury or hearing loss. The location of the IAC can be found by locating the arcuate eminence (beneath which is the superior semicircular canal) or by locating the superior petrosal nerve, which leads to the geniculate ganglion of the facial nerve. The IAC superiorly is drilled from medial to lateral with special care laterally so as not to violate the superior semicircular canal, labyrinthine portion of the facial nerve, and the basal turn of the cochlea. These structures are the lateral limits of the exposure and are within 2 to 3 mm of each other. The labyrinthine portion of the facial nerve comes laterally from the lateral end of the IAC to the geniculate ganglion at a 45-degree angle, is not covered by dura, and is quite small. This is the portion of the facial nerve most commonly injured during middle fossa removal of an acoustic tumor.
Advantages include better hearing preservation, low incidence of CSF leak and headache, excellent exposure to the lateral portion of the IAC, and less meningeal irritation. The dura does not need to be opened because this protects the temporal lobe from further injury. Disadvantages are a higher incidence of CN7 injury, occasional memory disturbances and aphasia, and seizures.
Because of limited access to the CPA, the middle cranial fossa approach is usually used for AS less than 2 cm in size, preferably those confined to the IAC. This approach may be combined with the RS approach for large tumors and is useful for lesions involving the cephalad portion of the petrous apex or junction of CN7 with the greater superficial petrosal nerve and clival region.
Complications
Complications related to AS resection are uncommon but include hemorrhage, stroke, vascular injury, infection and meningitis, brainstem and cranial nerve injury, CSF leak, and death. The incidence of cranial nerve injury has diminished with the availability of real-time monitoring. CN7 in particular can be difficult to identify secondary to displacement and effacement, adhesions, or involvement by the tumor.
CSF leaks may occur with all three approaches, especially if a combined approach is used. Air cells opened during the craniotomy or drilling around the IAC need to be sealed by bone wax or fascia. Leaks may resolve with conservative measures, such as elevation of the head and lumbar drains. Occasionally surgery is required, especially if the patient experiences meningitis. Over a 2- to 3-month basis, in patients with CSF leak, meningitis occurs 50% of the time. Perioperative meningeal irritation is common and managed conservatively.
Postoperative Imaging
The need for postoperative imaging is dictated by the tumor pathology and completeness of resection. For benign lesions, a baseline postoperative examination 6 to 12 months after surgery is useful. Some advocate follow-up imaging (for AS) 5 years after surgery, assuming the patient is asymptomatic. Unfortunately, because the ear may be “dead,” the first sign of recurrence could be brainstem compression. If imaged 1 to 2 years after surgery it may be difficult to differentiate postoperative change from recurrent or residual disease without a baseline examination. This commonly leads to unnecessary multiple additional follow-up examinations to clarify and creates significant anxiety for patients.
The baseline examination after uncomplicated surgery may be confined to the posterior fossa (unless otherwise indicated). For these examinations a suggested protocol is axial 2-mm T1-weighted images precontrast and postcontrast along with thin T2* weighted images (FIESTA, CISS, and so forth). Diffusion sequences are useful if the original tumor was an epidermoid. Precontrast images are useful for definition of fat and hemorrhage. Fat saturation sequences are useful but uneven fat saturation is common and pathology may be missed. On subsequent examinations, only the T2* and postcontrast T1 images need to be obtained. These limited examinations save a considerable amount of money over the patient’s lifetime as compared with complete brain and IAC examinations.
CT is less useful for following patients after AS surgery but excludes large tumors in patients where MR imaging is contraindicated. For bone-destroying tumors, subtle destruction of additional bone demonstrated by CT is more sensitive for detection of disease progression than is change of tumor size detected by MR imaging.
Following translabyrinthine surgery, there is a roughly triangular defect in the mastoid extending anteriorly through the labyrinth, the apex directed toward the IAC. Portions of the labyrinth may remain anteriorly. Signal in the residual labyrinth may be altered by blood, fibrosis, or ossification. On MR imaging, the fat graft filling the defect is the predominant feature early on but may resorb or atrophy over time ( Fig. 15 ). Fascia closing the dural defect is typically poorly visualized.