Imaging evaluation of the postoperative orbit remains challenging even for the expert neuroradiologist. This article provides a simplified framework for understanding the complex postoperative appearances of the orbit, in an attempt to enhance the diagnostic accuracy of postoperative computed tomography and MR imaging of the orbit. Readers are familiarized with the normal appearances of common eye procedures and orbit reconstructions to help avoid interpretative pitfalls. Also reviewed are imaging features of common surgical complications, and evaluation of residual/recurrent neoplasm in the setting of oncologic imaging surveillance.
Key points
- •
Inappropriate neuroimaging preceding ophthalmology referral can lead to inappropriate use of costly resources, with a potential for overdiagnosis and false positives.
- •
Imaging plays a critical role in recognizing the normal postoperative appearance of the commonly encountered orbital procedures, and enables early diagnosis of complications.
- •
The common eye surgeries that are evident on imaging include: (1) cataract surgery and intraocular lens implant; (2) retinal detachment surgeries (retinopexy, scleral buckling, and vitrectomy); (3) glaucoma aqueous shunting; (4) evisceration and enucleation; (5) ocular prosthesis; (6) eyelid weights; (7) dacryocystorhinostomy; (8) orbital decompression; (9) maxillofacial orbital reconstruction; and (10) orbital exenteration and reconstruction.
- •
Silicone oil, silicone sponge and rubber band, prosthesis, and implant should not be mistaken for unintended foreign bodies. Postoperative infection should be promptly identified to avoid the devastating consequence of permanent loss of vision.
- •
Oncologic orbital reconstruction uses vascularized free flaps composed of skin, subcutaneous fat, or fascia, with or without muscle, and at computed tomography and MR imaging their appearance reflects the flap compositions. The expected swelling and enhancement of a normal flap as a result of denervation or adjuvant chemoradiation should not be mistaken for neoplasm. Neoplasm tends to recur at the interface of the free flap and recipient surgical bed, and demonstrates mass-like growth with an appearance similar to that of the original tumor.
Introduction
The Centers for Disease Control and Prevention report the leading causes of blindness and low vision in the United States as age-related eye diseases such as macular degeneration, cataract, diabetic retinopathy, and glaucoma. Cataract is the leading cause of blindness and can occur at any age. An estimated 20.5 million (17.2%) Americans aged 40 years and older have cataract, and 6.1 million require intraocular lens (IOL) replacement. Diabetic retinopathy is the leading cause of blindness in American working-aged adults (20–74 years of age) with an estimated 4.1 million Americans affected by the disease. Vitrectomy plays a vital role in the management of severe complications of diabetic retinopathy. Glaucoma is the second leading cause of blindness worldwide in Caucasians, and the leading cause of blindness in Africans and Latinos. In all ethnic groups, the prevalence of glaucoma increases dramatically with age, and the aging population in the United States is expected to increase by an additional 67% by 2050. As a result, the number of persons with glaucoma is expected to increase an additional 50% from 2000 to 2020, leading to increasing utilization of aqueous shunting procedures. The number of patients with postoperative orbital procedures encountered on head and neck imaging will also be increased because of the increasing epidemic of diabetes and our rapidly aging population.
A variety of primary and secondary tumors affecting the orbits (retinoblastoma, rhabdomyosarcoma, uveal melanoma, orbital invasion from sinonasal and skin malignancies, or metastatic disease) require various treatment algorithms. The data extracted from the Surveillance, Epidemiology, and End Results database show that the best relative survival of the common orbital neoplasms, including uveal melanoma, lacrimal gland tumor, and sinonasal malignancies with orbital involvement, was noted in patients treated with surgery or a combination of surgery and chemoradiotherapy. Given the multidisciplinary medical advancements in treating orbital neoplasms, there is an increasing number of survivors who require surveillance imaging to monitor local disease control and treatment response, and to detect early recurrence and guide subsequent management.
Other common orbital procedures include orbital decompression for thyroid orbitopathy and maxillofacial orbital reconstruction in the setting of trauma. This article is not meant to be encyclopedic, and is intended only to provide pertinent information relevant to the imaging evaluation of the commonly encountered orbital procedures, outlined in Table 1 .
| Pathology | Surgery | Therapeutic Goals | Highlights of Surgical Technique |
|---|---|---|---|
| Cataract | Intraocular lens implant (IOL) | To replace the damaged lens to restore vision | IOL replaces the surgically removed cataractous lens |
| Glaucoma | Glaucoma shunt implant | To reduce intraocular pressure by decompression of aqueous humor | Glaucoma shunt is implanted most commonly in the superior temporal quadrant of the globe |
| Retinal detachment | Retinopexy Scleral buckling Vitrectomy | To reappose rhegmatogenous and or tractional retinal detachment | Scleral buckle is placed radially, segmentally, or circumferentially around the globes Intraocular injected air or silicone oil fills the vitrectomy cavity |
| Unsalvageable globe from trauma or infection or ocular tumor | Evisceration, enucleation and ocular implant | To eradicate the disease and provide cosmesis | Removal of the globe without (evisceration) or with (enucleation) removal of the sclera. Ocular implant is placed into the empty socket |
| Eyelid paralysis | Eyelid weight implant | To close the upper eyelid | Subcutaneous gold implant is secured to the tarsus in upper eyelid |
| Lacrimal obstruction | Dacryocystorhinostomy | To relieve lacrimal obstruction | Stent drains the lacrimal fossa to the inferior meatus |
| Thyroid-associated orbitopathy | Orbital decompression | To decompress the orbit for cosmesis and compressive optic neuropathy | Surgery removes the medial, inferior, or lateral orbital walls |
| Fracture | Orbital wall reconstruction | To restore orbital volume, cosmesis, and function | Autologous bone or cartilage, silicone, titanium plate and mesh, porous polyethylene material are used to reconstruct the orbital walls |
| Orbit malignancy | Orbital exenteration and reconstruction | To resect tumor and close orbital exenteration defect and restore cosmesis | Free flap is contoured to fill the exenteration defect and its vascular pedicle is reanastomosed to the available regional vessels |
Given a gamut of orbital procedures and surgeries performed for a variety of clinical indications with a range of clinically acceptable therapeutic efficacy, the imaging interpretation of the postoperative orbit is more likely to be inaccurate if performed outside the clinical context. In a symptomatic patient, the imaging may detect a new or progressive abnormality, more likely to alter the treatment, and hence contribute to the patient’s well-being. On the other hand, in an asymptomatic patient, overdiagnosis with false-positive findings can induce additional workup leading to increased morbidity, anxiety, and downstream costs. For these reasons, the clinical importance of postoperative imaging of the orbit relies on the radiologist’s confidence in diagnosing the normal postoperative appearance, to unequivocally identify procedural complications and detect early neoplastic recurrence in oncologic surveillance.
Introduction
The Centers for Disease Control and Prevention report the leading causes of blindness and low vision in the United States as age-related eye diseases such as macular degeneration, cataract, diabetic retinopathy, and glaucoma. Cataract is the leading cause of blindness and can occur at any age. An estimated 20.5 million (17.2%) Americans aged 40 years and older have cataract, and 6.1 million require intraocular lens (IOL) replacement. Diabetic retinopathy is the leading cause of blindness in American working-aged adults (20–74 years of age) with an estimated 4.1 million Americans affected by the disease. Vitrectomy plays a vital role in the management of severe complications of diabetic retinopathy. Glaucoma is the second leading cause of blindness worldwide in Caucasians, and the leading cause of blindness in Africans and Latinos. In all ethnic groups, the prevalence of glaucoma increases dramatically with age, and the aging population in the United States is expected to increase by an additional 67% by 2050. As a result, the number of persons with glaucoma is expected to increase an additional 50% from 2000 to 2020, leading to increasing utilization of aqueous shunting procedures. The number of patients with postoperative orbital procedures encountered on head and neck imaging will also be increased because of the increasing epidemic of diabetes and our rapidly aging population.
A variety of primary and secondary tumors affecting the orbits (retinoblastoma, rhabdomyosarcoma, uveal melanoma, orbital invasion from sinonasal and skin malignancies, or metastatic disease) require various treatment algorithms. The data extracted from the Surveillance, Epidemiology, and End Results database show that the best relative survival of the common orbital neoplasms, including uveal melanoma, lacrimal gland tumor, and sinonasal malignancies with orbital involvement, was noted in patients treated with surgery or a combination of surgery and chemoradiotherapy. Given the multidisciplinary medical advancements in treating orbital neoplasms, there is an increasing number of survivors who require surveillance imaging to monitor local disease control and treatment response, and to detect early recurrence and guide subsequent management.
Other common orbital procedures include orbital decompression for thyroid orbitopathy and maxillofacial orbital reconstruction in the setting of trauma. This article is not meant to be encyclopedic, and is intended only to provide pertinent information relevant to the imaging evaluation of the commonly encountered orbital procedures, outlined in Table 1 .
| Pathology | Surgery | Therapeutic Goals | Highlights of Surgical Technique |
|---|---|---|---|
| Cataract | Intraocular lens implant (IOL) | To replace the damaged lens to restore vision | IOL replaces the surgically removed cataractous lens |
| Glaucoma | Glaucoma shunt implant | To reduce intraocular pressure by decompression of aqueous humor | Glaucoma shunt is implanted most commonly in the superior temporal quadrant of the globe |
| Retinal detachment | Retinopexy Scleral buckling Vitrectomy | To reappose rhegmatogenous and or tractional retinal detachment | Scleral buckle is placed radially, segmentally, or circumferentially around the globes Intraocular injected air or silicone oil fills the vitrectomy cavity |
| Unsalvageable globe from trauma or infection or ocular tumor | Evisceration, enucleation and ocular implant | To eradicate the disease and provide cosmesis | Removal of the globe without (evisceration) or with (enucleation) removal of the sclera. Ocular implant is placed into the empty socket |
| Eyelid paralysis | Eyelid weight implant | To close the upper eyelid | Subcutaneous gold implant is secured to the tarsus in upper eyelid |
| Lacrimal obstruction | Dacryocystorhinostomy | To relieve lacrimal obstruction | Stent drains the lacrimal fossa to the inferior meatus |
| Thyroid-associated orbitopathy | Orbital decompression | To decompress the orbit for cosmesis and compressive optic neuropathy | Surgery removes the medial, inferior, or lateral orbital walls |
| Fracture | Orbital wall reconstruction | To restore orbital volume, cosmesis, and function | Autologous bone or cartilage, silicone, titanium plate and mesh, porous polyethylene material are used to reconstruct the orbital walls |
| Orbit malignancy | Orbital exenteration and reconstruction | To resect tumor and close orbital exenteration defect and restore cosmesis | Free flap is contoured to fill the exenteration defect and its vascular pedicle is reanastomosed to the available regional vessels |
Given a gamut of orbital procedures and surgeries performed for a variety of clinical indications with a range of clinically acceptable therapeutic efficacy, the imaging interpretation of the postoperative orbit is more likely to be inaccurate if performed outside the clinical context. In a symptomatic patient, the imaging may detect a new or progressive abnormality, more likely to alter the treatment, and hence contribute to the patient’s well-being. On the other hand, in an asymptomatic patient, overdiagnosis with false-positive findings can induce additional workup leading to increased morbidity, anxiety, and downstream costs. For these reasons, the clinical importance of postoperative imaging of the orbit relies on the radiologist’s confidence in diagnosing the normal postoperative appearance, to unequivocally identify procedural complications and detect early neoplastic recurrence in oncologic surveillance.
Important anatomic and functional considerations
Vision is the most valuable sense, requiring an optimal and synchronized function of the eyelid, globe, lens, retina, and optic nerve, in addition to extraocular muscles and neurovascular structures. The commonly performed orbital procedures aim to protect and preserve the primary orbital function as a visual organ and the secondary function as the center of facial cosmesis.
Globe
To facilitate the understanding of the surgical principle of ocular surgeries, the anatomy of the globe is briefly discussed. There are 3 fluid-filled spaces in the globe: the anterior chamber, the posterior chamber, and the vitreous cavity. The anterior chamber extends from the cornea to the iris and communicates, through the pupil, with the posterior chamber. The posterior chamber extends from the posterior surface of the iris to the anterior surface of the vitreous. The ciliary body, which produces the aqueous humor, and the intraocular lens are located in the posterior chamber. The anterior and posterior chambers are filled with aqueous humor. The vitreous cavity occupies the posterior globe behind the posterior chamber and contains gel-like vitreous humor.
The sclera, uvea, and retina compose the 3 layers of the globe. The sclera is a fibrous capsule around the globe contiguous with the cornea anteriorly and the dural sleeve of the optic nerve posteriorly. The uvea consists of the iris and the ciliary body anteriorly and the choroid layer posteriorly. The retina comprises the sensory retina layer supported by the retinal pigment epithelium beneath, all nourished by the choroid. Conjunctiva lines the inside of the eyelids and covers the anterior sclera.
Orbit
Orbital fractures with large floor defects may cause enophthalmos. On the other hand, increase of the intraorbital fat and enlargement of the extraocular muscles from thyroid orbitopathy result in exophthalmos, with stretching and/or compression of the optic nerve, resulting in visual compromise. At the tight orbital apex, the optic nerve is surrounded by the annulus of Zinn, rendering the optic nerve vulnerable to compression by even a small mass lesion or extraocular muscle enlargement, or by osseous narrowing. Therefore, the general role of reconstructive or decompressive surgeries of the orbit is to restore the normal orbital volume to preserve its function and cosmesis.
Imaging protocols
The acquisition of retinal images largely depends on fundoscopic examination and fundus photography. Ultrasonography has become an indispensable imaging adjuvant for the clinical assessment of a variety of ocular diseases by the ophthalmologist. However, many such techniques are constrained by a relatively small field of view, and are often limited when there is disease-induced opacification of the lens or vitreous hemorrhage. Even though other imaging techniques such as computed tomography (CT) or MR imaging are more often not the first test of choice in diagnosing the common ocular disorders, these offer the ability to acquire images despite media opacification. These imaging tools are also valuable in evaluating the deep orbital structures behind the globe to define the full extent of the clinically evident infection, inflammation, or neoplasm, or in cases of blunt trauma.
CT is the workhorse in imaging evaluation for calcification, infection, integrity of the implants, and orbital reconstruction. The strength of CT is in evaluating the integrity of the osseous structure and the reconstructive hardware; 3-dimensional (3D) volume rendering provides valuable assessment for the restoration of the orbital contour and volume. The superb soft-tissue characterization of MR is best used in oncologic surveillance. General guidelines for the recognition of the normal imaging appearance of commonly used materials for routine ophthalmologic surgeries are suggested in Table 2 .
| Procedure | Materials | Normal Imaging Findings |
|---|---|---|
| Intraocular lens implant (IOL) | Acrylic, silicone, or polymethyl methacrylate | Absence of normal lens Most IOLs are linear, thin, hyperattenuating optics placed between anterior and posterior chambers |
| Glaucoma shunt implant | Silicone, polypropylene, or polyethylene | The plate of the shunt is most commonly implanted on the superotemporal quadrant of the globe Silicone is homogeneously hyperdense; polypropylene is homogeneously hypodense All show low signal intensity on MR imaging A small bleb (fluid pocket) may be seen at the implant |
| Scleral buckling | Silicone solid rubber band, silicone sponge | The buckle may normally indent the globe Silicone rubber band shows hyperattenuating band encircling the globe. Silicone sponge shows air-density wedge on the globe |
| Ocular injection | Air, gas, silicone oil | Air and gas have air attenuation. Silicone oil appears homogeneously hyperattenuating |
| Ocular implant and eye prosthesis | Medpor a polyethylene, acrylic, silicone, hydroxyapatite | Medpor has hypodensity between fluid and fat. Both acrylic and silicone are homogeneously hyperattenuating Hydroxyapatite is very hyperdense with characteristic lattice pattern |
| Eyelid weights | Gold | Metallic plate is imbedded in the upper eyelid |
| Orbital reconstruction | Autologous bone graft, titanium fixation pin, plate, mesh, and porous polyethylene | Bone graft or metallic hardware is contoured to the orbital defect using osteotomies, plates, and pins Porous polyethylene has hypodensity or soft-tissue density |
| Orbital exenteration and free flap reconstruction | Fasciocutaneous flap Myocutaneous flap | The fatty component demonstrates fat signal characteristic on T1-weighted image The muscular component has characteristic muscular striation, T1 isointensity to muscle, and variable T2 signal intensity, and exhibits variable enhancement |
Depending on the orbital subsites, the treated abnormality, and the clinical concern following surgical procedures, CT or MR imaging is tailored to provide high-resolution details of the orbit alone or to evaluate the regional orbital operative bed including the surrounding maxillofacial and skull base structures ( Box 1 ).
CT orbit
Noncontrast examination a
Axial with multiplanar reformats (3 mm thick)
Three-dimensional volumetric rendering of the bone is useful in evaluating orbital reconstruction
Field of view includes the orbits and extends from the tip of the nose to the brainstem
MR imaging orbit
Noncontrast sequences followed by contrast-enhanced sequences:
Axial DW imaging and T2-weighted FSE/TSE with fat saturation of entire head (5 mm thick). Axial and coronal T1-weighted FSE/TSE, coronal STIR, or T2-weighted FSE/TSE with fat saturation (3 mm thick). Gadolinium-enhanced axial and coronal T1-weighted spin echo with fat saturation (3 mm thick)
Field of view extends from the top of orbits to 2 cm below hard palate and from tip of nose to brainstem.
Abbreviations: DW, diffusion-weighted; FSE, fast spin echo; STIR, short-tau inversion recovery; TSE, turbo spin echo.
a Contrast-enhanced CT can be performed to evaluate venous structures and enhancement of the lesion.
Urgent postoperative complications and imaging pitfalls
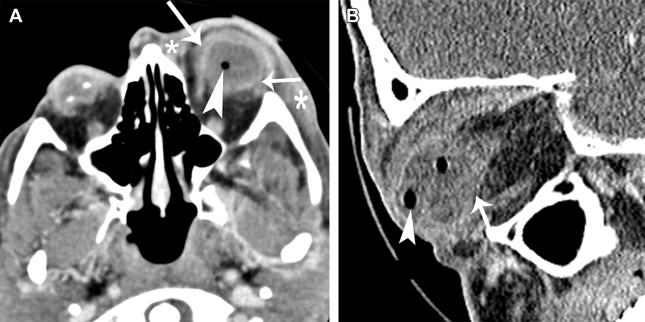
Most postoperative complications are diagnosed clinically by the ophthalmologist. Endophthalmitis is acute suppurative infection of the globe, which may occur acutely after an ophthalmologic procedure (within 6 weeks of surgery) or follow an indolent course with delayed onset (more than 6 weeks after surgery). Clinically it is characterized by ciliary injection, conjunctival chemosis, decreased visual acuity, and ocular pain. Characteristic imaging of acute fulminant infection and progression of disease should not be mistaken for normal surgical sequelae ( Figs. 1 and 2 ). Although procedure-related retinal detachment is mostly diagnosed clinically, new retinal and choroidal detachments may herald an intraocular tumor ( Fig. 3 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree