Small bowel tumors are rare gastrointestinal tumors. Neuroendocrine tumors are the most common and demonstrate unique subtypes depending on their location. Adenocarcinomas are most common in the duodenum demonstrating luminal narrowing and irregularity. Gastrointestinal stromal tumors are heterogeneously enhancing lesions with endophytic and/or exophytic growth patterns. Immunotherapy is a unique treatment of these tumors with tumoral response best assessed with both routine computed tomography (CT) and PET/CT. Primary small bowel lymphoma has many imaging patterns, most commonly being aneurysmal dilation and thickening of the small bowel. Metastases are common and may present as polypoid lesions, focal wall thickening, or serosal deposits.
Key points
- •
Computed tomography and MR enterography are the imaging modalities of choice for evaluating small bowel tumors.
- •
Neuroendocrine neoplasms are small hyperenhancing lesions that are often multifocal within the small bowel.
- •
Small bowel adenocarcinomas are usually solitary annular/semi-annular masses with abrupt shouldering and luminal narrowing.
- •
Gastrointestinal stromal tumors are variably sized, smoothly marginated, lobulated tumors often with necrosis, hemorrhage, and/or degeneration.
- •
Primary small bowel lymphomas vary in imaging appearance and may be nodular/polypoid, infiltrative, or endoexoenteric with cavitation and fistulation common.
Introduction
Small bowel tumors account for only 5% of gastrointestinal (GI) tumors in the United States but have been rising in incidence over recent decades. The proportion of neuroendocrine tumors (NETs) has steadily increased since the 1970s, partly due to the increased use of cross-sectional imaging and improved small bowel imaging techniques. Imaging plays a crucial role in detecting and diagnosing small bowel tumors, enhancing the ability to identify and treat various malignancies at earlier stages. Diagnosing small bowel tumors is challenging as many patients are asymptomatic or present with nonspecific symptoms such as abdominal pain, weight loss, nausea, vomiting, intestinal obstruction, and GI bleeding. , This article explores the current imaging techniques for small bowel evaluation, focusing on computed tomography (CT) and MR enterography, and examines their role in the 5 most common small bowel malignancies: neuroendocrine neoplasms (NENs), adenocarcinoma, gastrointestinal tumors (GISTs), small bowel lymphoma, and metastases.
Imaging techniques
Traditionally, fluoroscopic small bowel follow-through studies and enteroclysis were primary imaging modalities for the small bowel, but noninvasive CT and MR imaging have largely replaced these techniques. CT offers rapid image acquisition, wide availability, consistent image quality, high spatial resolution, and multi-planar reformatting capabilities. MR imaging provides multi-timepoint imaging, superior contrast resolution, and lacks ionizing radiation. Both modalities assess the lumen, mural thickness, extramural extent, and extraintestinal findings. Routine abdominopelvic CT without oral contrast and sufficient bowel distension is not optimal for small bowel assessment, with studies showing routine single-phase portal venous CT frequently missing small bowel tumors compared to CT enterography (CTE). One study found a CTE small bowel tumor detection rate of 95% versus only 45% with routine single-phase CT. , Thus, CT and MR enterography (MRE) with optimal small bowel distension have become the mainstay for small bowel imaging.
For both CTE and MRE, patients fast for 4 to 6 hours before the examination to enhance compliance with drinking a large volume of fluid and to reduce intraluminal filling defects that can mimic masses. Patients ingest approximately 1 L of oral contrast material in split doses 60 minutes prior to scanning to distend the small bowel lumen ( Table 1 ). Commercially available contrast agents for enterography provide neutral contrast (similar to water) on CTE and biphasic characteristics on MRE (low T1 and high T2 signal intensity). For CTE, patients receive intravenous (IV) contrast material, and images are usually obtained during an enteric or early portal venous phase (50–60 seconds post-contrast injection). Images are acquired from the lung bases through the perineum with thin section acquisition and with multiplanar reformations created. Some small bowel tumors may be more conspicuous on different phases, although a single phase often suffices for most neoplasms. A multiphase protocol should be employed, however, when GI bleeding is suspected.
| Contrast | Protocol |
|---|---|
| Neutral enteric contrast—for example, Breeza, VoLumen, or CitraClear, allowing for adequate distension of small intestine | 500 mL PO 60 min before scan 500 mL PO 45 min before scan 500 mL PO 30 min before scan 500 mL PO water 15 min before scan ∗ NPO for 4–6 h prior to scan |
| Intravenous contrast | CT: Weight-based dosing of Omnipaque 300 followed by 50 mL of 0.9% NaCl MR imaging: Weight-based dosing of Gadavist: 1 mmol/mL |
| CT Phases |
|
| MR Sequences |
|
∗ The ∗ is an additional note that patients need to be NPO 4-6 hours prior to scan as part of the enterography protocol.
The oral contrast protocol for MRE mirrors that of CTE. Administering spasmolytics (IV or intramuscular [IM] Glucagon or IV or sublingual hyoscine butylbromide) improves image quality for MRE, which is more sensitive to motion artifacts. MR images are obtained both before and after IV gadolinium contrast administration, with multi-planar imaging performed in both the axial and coronal planes. MR imaging may also be performed in the prone position to reduce motion artifact and improve small bowel distension. Motion-insensitive T2-weighted images (single shot fast spin echo [SSFSE], balanced steady-state free precession [bSSFP]) provide high contrast between the high T2 signal intensity oral contrast and relatively lower T2 signal intensity masses. The bSSFP sequences offer more homogenous intraluminal fluid compared to the SSFSE images, which suffer from intraluminal flow void artifacts. Diffusion-weighted images (DWIs) may help identify subtle lesions or confirm suspicious findings by demonstrating restricted diffusion. Three dimensional (3D) T1-weighted images performed before IV contrast administration identify high signal intensity intraluminal material or blood that can mimic enhancement. Post-contrast 3D T1-weighted images improve the detection of enhancing small bowel lesions, and multiphasic acquisition can mitigate equivocal findings secondary to bowel peristalsis and other imaging artifacts. Table 1 provides CTE and MRE technique parameters.
Endoscopic evaluation
Conventional endoscopic techniques provide a detailed evaluation of the mucosal surface with the capability for biopsy but have limited reach into the small bowel. Balloon-assisted enteroscopy (antegrade or retrograde) allows access to a greater length of the small bowel but is time-consuming and requires specialized expertise. Video capsule endoscopy (VCE) offers a comprehensive view of the entire small bowel but presents challenges in localization and does not permit biopsy. Known or suspected GI obstruction is an absolute contraindication for VCE due to risk of retention, which may require surgical retrieval. Endoscopic techniques also have limitations in detecting submucosal tumors and cannot provide information about the extramural extent of small bowel tumors. Studies show that CTE is more sensitive (92.7%) than capsule endoscopy (29.6%) in detecting small bowel tumors. ,
Histology
The small bowel consists of 4 layers: the mucosa, submucosa, muscularis propria, and serosa ( Fig. 1 ). The mucosa, the inner-most layer, contains the epithelium, lamina propria, and muscularis mucosa. The mucosa features villi, finger-like projections extending into the bowel lumen, lined by the epithelial layer consisting of absorptive enterocytes and secretory Goblet cells and with a core connective tissue layer called the lamina propria. The crypts of Lieberkuhn, the deepest part of the epithelium, contain enteroendocrine cells. The submucosa includes connective tissue, blood vessels, lymphatics, and the Meissner plexus. Peyer patches are lymphoid follicles located in the lamina propria and submucosa of the ileum. The muscularis propria is responsible for peristalsis. It contains the myenteric (Auerbach) nervous plexus between its 2 muscular layers and also houses the interstitial cells of Cajal, the pacemakers for peristalsis. The serosa, the outermost layer present only in intraperitoneal segments, secretes serous fluid for lubrication.

Small bowel tumors
Neuroendocrine
NENs are the most common primary small bowel malignancy. They originate from intraepithelial enteroendocrine cells and can occur throughout the small bowel. Functional NENs secrete hormones causing specific syndromes, whereas nonfunctional NETs do not secrete clinically significant amounts of hormones. For this review, small bowel NENs are defined as those arising between the ligament of Treitz and the ileocecal valve, the majority of which occur in the distal ileum. Most are nonfunctional, slow-growing, and often incidentally detected. , The World Health Organization classifies NENs into well-differentiated NETs (80%–90%), poorly differentiated neuroendocrine carcinomas (NECs, 10%–20%), and mixed neuroendocrine neoplasms (MiNENs)/non-NENs based on mitotic rate, Ki-67 index, and degree of differentiation. NECs have distinct molecular mutations and are subdivided into small-cell and large-cell subtypes, both with high mitotic rate and Ki-67 expression ( Table 2 ). MiNENs, with histologic features of both neuroendocrine and non-NETs, carry a poor prognosis.
| WHO Category | Tumor Grade | Degree of Differentiation | Mitotic Rate (Mitoses/2 mm 2 ) | Ki-67 Index (%) |
|---|---|---|---|---|
| Neuroendocrine Tumors (NET) | Low (Gr 1) | Well | <2 | <3 |
| Intermediate (Gr 2) | Well | 2–20 | 3–12 | |
| High (Gr 3) | Well | >20 | >20 | |
| Neuroendocrine Carcinoma (NEC) | High, small cell type (SCNEC) | Poorly | >20 | >20 |
| High, Large cell type (LCNEC) | Poorly | >20 | >20 | |
| Mixed Neuroendocrine-Nonneuroendocrine Neoplasm | Variable | Well or Poorly | Variable | Variable |
Over half of small bowel NENs are detected by imaging, with smaller percentage identified at endoscopy or surgery. Primary small bowel NENs are often small but hypervascular, appearing as hyperenhancing lesions on both CT and MR imaging during the arterial and/or enteric phase ( Fig. 2 A, B ). They are multifocal in up to 33% to 54% of patients. Most lesions are less than 2 cm and they can have a variety of morphologies: polypoid mucosal or submucosal lesion, eccentric plaque-like mural thickening (often with a crescentic appearance) with or without serosal puckering, and less commonly, carpet-like lesions with segmental submucosal spread. MR imaging shows small bowel NENs as T1 isointense, T2 isointense or hyperintense, with avid arterial enhancement, and restricted diffusion. Both CTE and MRE provide high per-patient sensitivity; however, per-lesion sensitivity suffers due to multifocality. The desmoplastic reaction of these tumors often causes kinking or obstruction of the small bowel

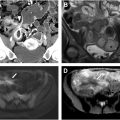
Despite their small size, NENs frequently metastasize, with nodal metastases common even in tumors less than 1 cm. Imaging often shows hyperenhancing nodal metastases, which may appear round and smooth or stellate with spiculation, calcification, and mesenteric retraction due to hormone production and secondary fibrosis ( Fig. 3 A, B ). Vascular encasement is common ( Fig. 4 A, B ). Extranodal metastases commonly occur in the liver but can also develop in the lungs, bones, ovaries, and peritoneum. Sensitivity for liver metastases increases with biphasic contrast-enhanced liver CT or multiphasic MR imaging as they often hyperenhance. Some radiologists advocate for hepatocyte-specific MR contrast agents for evaluating hepatic metastases. Liver metastases are usually conspicuous and hyperintense on MR T2-weighted sequences and DWI.


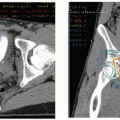
PET/CT or PET/MR imaging with somatostatin receptor analogs exploits the overexpression of somatostatin, present in 80% to 100% of small bowel NENs ( Fig. 4 C). They offer high per-patient sensitivity (but a much lower per-lesion sensitivity) and specificity for a primary tumor and metastases and can potentially help guide peptide receptor radionuclide therapy. Somatostatin receptor PET/CT is less useful for well-differentiated NENs; however, it may have a role in poorly differentiated NENs. Up to 10% of patients with stage IV NEN develop carcinoid syndrome, caused by the release of serotonin and other vasoactive substances, leading to symptoms like flushing, diarrhea, wheezing, and, in severe cases, heart valve lesions.
Adenocarcinoma
Small bowel adenocarcinomas make up about approximately 31% to 40% of small bowel malignancies. These primary adenocarcinomas, arising from the glandular epithelium, are most common in the duodenum (60%), followed by the jejunum (25%–29%), and ileum (10%–13%). Patients often present with nonspecific symptoms, leading to delayed diagnoses and many patients present emergently due to obstruction or bleeding from advanced disease. Most cases are sporadic, but associations with polyposis syndromes and inflammatory bowel diseases like Crohn’s or celiac disease exist.
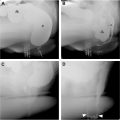
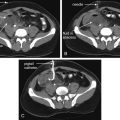
Small bowel adenocarcinomas likely arise via an adenoma-to-carcinoma transformation, similar to colorectal carcinomas. The morphology (tubular/tubulovillous/villous), size, location, and multicentricity of the adenomas are significant risk factors for malignant transformation ( Fig. 5 A, B ). , Imaging findings vary by location with periampullary and duodenal tumors, most common in the setting of polyposis syndromes, often appearing more polypoid and well-circumscribed. However, many small bowel adenocarcinomas present as solitary annular or semi-annular masses with abrupt shouldering and luminal narrowing (apple-core morphology; Fig. 6 A ). , Ulceration is common, and luminal narrowing often results in small bowel obstruction ( Fig. 6 B). Most have mild homogeneous or heterogeneous contrast enhancement with increasing size. MR imaging shows adenocarcinomas as mildly T2 hyperintense lesions with restricted diffusion. Locally advanced lesions may infiltrate into the adjacent mesenteric fat ( Fig. 6 C). Many cases present with regional lymph node metastases and distant metastases, commonly in liver and peritoneum. ,