Medical imaging serves first to diagnose disease or injury, but imaging techniques are increasingly used to guide temporizing or definitive therapy for a range of conditions. Depending on the practice setting, medical treatments guided by imaging techniques may be performed by interventional radiologists, cardiologists, and surgeons. In many cases, emergency physicians perform image-guided procedures, using ultrasound to guide procedures as diverse as peripheral and central venous catheter placement, abdominal paracentesis, thoracentesis, lumbar puncture, and joint aspiration. Fluoroscopic guidance of fracture reduction is a standard practice for orthopedists and many emergency physicians.
In this chapter, we discuss some selected applications of image-guided procedures, as well as the evidence supporting their use. At their best, image-guided procedures are definitive treatment, with strong evidence suggesting equivalent or superior clinical outcomes compared with other medical and surgical approaches. In other cases, the evidence disappoints the medical community, showing no benefit to a therapy with great initial expectations. Many applications of image-guided therapies have surprisingly little evidence to support them, despite having been widely accepted by the medical community. Other applications are rarely used today but offer “heroic” options in moribund patients with few treatment options for their life-threatening conditions. The emergency physician must be familiar with the available interventions, their indications and contraindications, and the class of supporting evidence.
We begin with a brief overview of imaging modalities used to guide procedures, with their advantages and disadvantages. We then discuss some important methodologic issues to consider when reviewing the evidence for any treatment, including image-guided therapies. Although most of us may never design or conduct research, we all must be capable of understanding research and recognizing when research is so seriously flawed that we can no longer trust the conclusions drawn by investigators. We provide some basic background so that we can draw appropriately limited conclusions from flawed research when possible. Finally, with this background, we grapple with some important image-guided procedures, comparing those with excellent evidence to those with more modest support.
Imaging Modalities for Procedures
Common imaging modalities used to guide procedures are listed in Table 16-1 . Ultrasound is commonly used to guide procedures because it has the benefit of allowing real-time visualization with no radiation exposure to the patient or medical provider. Plain x-ray can be used to guide a procedure but has the disadvantage of radiation exposure to the patient and a lack of real-time guidance. Instead, a series of x-ray images taken at intervals is used to assess the progress of the procedure. An example is the use of repeated x-rays to assess the success of a fracture reduction. Fluoroscopy (a video x-ray technique) is frequently used for procedural guidance, with the advantage of real-time guidance (providing the opportunity for both “live video” and “stop-action snapshots” of the procedure). Fluoroscopy is the imaging modality employed in common catheter angiography procedures, and it allows visualization of bone and radiopaque contrast agents. Unfortunately, fluoroscopy provides a relatively high radiation dose to the patient, proportional to the amount of time that the fluoroscope tube is turned on. The exposure to the medical provider (and particularly the cumulative dose for physicians who routinely perform fluoroscopic exams) can also be relatively high if care is not taken to use shielding devices and to keep the practitioner’s body outside of the field of the fluoroscope. The radiation dose to the patient is relatively unimportant when the body part imaged is radiation insensitive (e.g., extremities, as during a fracture reduction). However, when the body region imaged is more radiosensitive (e.g., chest or abdomen), the total time of the imaging should be limited to reduce the radiation dose. CT provides an excellent imaging tool for procedural guidance, allowing a target structure such as an intraabdominal abscess to be precisely localized. Measurements of distance to the structure of interest are easily made with CT, and the locations of instruments such as trocars, guidewires, and catheters can be accurately determined. Continuous CT fluoroscopy allows near real-time guidance. Alternatively, in the “quick-check” technique, several images may be acquired over time, first to localize the lesion of interest and then to assess the progress of the procedure intermittently. This repeated imaging with CT results in a moderately high radiation dose in a focused area, though the cephalad–caudad extent of the repeated images is usually restricted only to the area of interest. The exposure to patients and radiologists from CT fluoroscopy can be minimized by low-milliampere CT technique and minimizing CT fluoroscopic time with the quick-check method.
| Imaging Modality | Radiation Dose | Real-Time Procedure Guidance? |
|---|---|---|
| Ultrasound | None | Yes |
| X-ray | Low | No ; intermittent images are obtained at intervals during the procedure |
| Fluoroscopy | Moderate to high, depending on total exposure time (during which the fluoroscope tube is on) | Yes |
| CT | Moderate to high, depending on number of CT images obtained * | Yes; CT fluoroscopy techniques allow near real-time imaging |
* In some cases, multiple CT images must be obtained to check the progress of the procedure, though the cephalad–caudad axis of the CT scan is usually limited.
Assessing the Quality of Scientific Evidence
In the sections that follow, we describe indications for image-guided procedures and briefly review the scientific evidence supporting the use of these procedures. In some cases, the evidence is quite limited, with case series describing the use of an intervention with no control group to allow comparisons with other treatments. In other scenarios, large prospective randomized-controlled studies allow more fair assessment of the clinical benefit of image-guided procedures, compared with other standard therapies, including medical treatments and surgical interventions.
Classes of medical evidence are summarized in Table 16-2. We refer to this hierarchy later in the chapter as we consider each application of an image-guided procedure. Here, we describe study design features that strongly influence the validity of results.
| Class of Evidence | Type of Study | Typical Features | Level of Recommendation |
|---|---|---|---|
| I | Randomized clinical studies using prospective data (or meta-analysis of the same) |
| A—high degree of clinical certainty |
| II | Nonrandomized trials or retrospective or case-control data |
| B—moderate degree of clinical certainty |
| III | Expert opinion or consensus; data from smaller, less-well-controlled studies or case series; or both |
| C—guarded, inconclusive, or preliminary recommendations |
Study Design Measures to Maximize Precision of Results
Studies that provide class I evidence include large populations of patients, providing the statistical power to answer the clinical question with narrow confidence intervals (CIs). This is in contrast to many initial studies, which are often small and may suggest an outcome benefit but with a broad range in the estimate of the benefit, sometimes including the possibility of no benefit or even harm because of the procedure. Small studies with broad CIs provide an imprecise estimate of the magnitude and direction of an effect. Large studies with narrow CIs provide precise estimates of magnitude and direction of effects. Whether these precise estimates reflect “truth” depends on the appropriate use of other design features to minimize bias, discussed later.
Study Design Measures to Allow Cause–Effect Determination
A second important feature of a study is the use of a comparison or control group. Without such a group, a cause–effect relationship cannot be determined for the intervention and the outcome of interest. We cannot know what the outcomes would have been if the intervention in question had not been performed. Instead, we can only state whether patients receiving the intervention did well or badly. This can overestimate or underestimate the value of intervention. For example, an intervention may have no effect, yet patients receiving it may do well clinically. The intervention might then be incorrectly credited with the good outcome, when other factors such as the severity of the original illness or the influence of other treatments may have been responsible.
Cause–effect determination also requires that the treatment be performed before the effect occurs. Although this may appear obvious, the lack of such a temporal relationship can be obscured in retrospective studies. For example, in a retrospective study, it may be that patients receiving a given intervention ultimately are seen to have better outcomes. Hidden from view may be some clinical feature that predicts better outcome and was already at play in those patients who were chosen for treatment; thus the intervention is actually a marker of that other unmeasured clinical factor rather than the cause of the clinical improvement. This effect is discussed in more detail later in our consideration of randomization. Prospective randomization is required to transform the relationship between intervention and outcome from a simple association or correlation to a likely cause–effect relationship. Because randomization is not possible in retrospective studies, prospective designs are necessary to prove causation. Retrospective studies can provide evidence of an association between an intervention and an outcome, suggesting the need for a prospective study to determine causation.
Study Design Measures to Minimize Bias and Increase the Credibility of Results
A third feature of reliable research is the use of measures to minimize bias. These include randomization, blinding, and representative study populations.
Randomization
Randomization is extremely important to the validity of a study. Randomization creates two (or more) equal groups before the intervention is performed. When randomization is performed and is ideal, the two groups start with similar conditions. This allows differences that are measured after the intervention to be attributed to the intervention, rather than to preexisting differences between the groups. Randomization would be less important if medical research involved identical subjects, sharing the same genetic composition and environmental background. Instead, human medical research subjects differ in a multitude of factors. The use of appropriate randomization attempts to balance these differences between the groups. Consider a clinical trial to be a bit like a race, with the measured outcome being to determine which of two race vehicles is faster or better. Randomization attempts to ensure that, on average, the drivers of the two race vehicles start at the same point and travel a racecourse of similar difficulty. Without this, it would be impossible to determine which vehicle is faster; one might travel a shorter or a longer distance along a straighter or a more tortuous course, with a different uphill or downhill grade.
The success of randomization in a trial can be loosely assessed by comparing the baseline characteristics of the two groups, usually reported in the first table of a published study. It cannot be assumed that randomization always works as intended. In large studies, randomization usually does distribute characteristics evenly between groups. However, in small studies, randomization often fails to achieve this goal. Consider the example of a coin flip with a fair coin. If the coin is flipped 1 million times, approximately the same number of heads and tails will occur. However, if the coin is flipped only 10 times, the number of heads and tails may differ—resulting in a random but unequal distribution of the study subjects before the intervention. By reviewing the distribution of characteristics of the study subjects, you can determine whether they differed at study inception in important measured ways, such as age, gender, race, the presence of medical conditions such as diabetes, or measures of disease severity (e.g., Acute Physiology and Chronic Health Evaluation scores). When the two study groups differ in one or more measured characteristics, beware of the possibility that any differences after the study intervention are the result of this starting difference, rather than because of the benefit or harm of the intervention. Apparent benefit may simply reflect that the subjects were healthier before the intervention. Apparent harm of the intervention may reflect that subjects receiving the intervention were sicker. Uneven distribution before the intervention can also hide a real effect. For example, consider an intervention group that was sicker at study inception despite randomization. If the two groups have similar results, it may appear that the intervention had no effect, when in reality it allowed the intervention group to overcome an initial disadvantage to achieve the same outcome as the control group. As you can see, randomizing patients to achieve two matched groups before any study intervention is essential to the credibility of the outcome. If groups differ at the beginning of the study, attempts to understand the cause–effect relationship of the intervention may be confounded.
When two groups appear similar on the basis of similar measured baseline characteristics after randomization, it remains possible that the two groups are not similar in other important but unmeasured variables. In large studies with good randomization, this is unlikely, but this possibility becomes important in small studies and in a common scenario in medical research—the statistical matching of groups. Researchers often perform statistical “corrections” to overcome either the lack of initial randomization, or the failure of randomization to perform as intended. Let’s consider both of these scenarios, because both can deceive us in our interpretation of research studies. In some studies, patients are not randomized before the study intervention is performed. This is common in retrospective studies, in which patients receive an intervention based on the decisions of the treating physician and their clinical status. As a consequence, patients in the treatment group often differ substantially from their counterparts who were treated differently. Because researchers would like to compare the outcomes in the two groups, they attempt to “correct” for these baseline differences, in effect trying to place the two groups at the same “starting line” in the “race.”
Often in observational studies without randomization, researchers match patients in both groups who share similar age, gender, and disease severity and then compare outcomes in these two “matched” groups. Although this may appear to serve the same function as prospective randomization, it differs in a key way. Using this “matching” strategy, the researchers sort patients into equal groups using measured characteristics, hoping that important unmeasured characteristics also sort in an equal way. This assumption may fail but be impossible to discern from the reported data. The most important baseline difference in the patients may not be a readily measured feature such as age or heart rate but, rather, the clinician’s judgment that one patient deserves aggressive therapy whereas another does not.
Consider this slightly absurd example: Two groups of patients are identified retrospectively, those who were treated with treatment A and those who received treatment B. Because these patients were not randomized to their treatment, they likely had some key difference that drove the physicians caring for them to choose treatment A or B. The researchers try to correct for this possible starting difference by matching patients in each group who are similar in age, height, gender, and initial disease or injury severity—perhaps measured by triage blood pressure. But the key characteristic that ultimately had the largest influence on patient outcome might have been some genetic factor, which in turn might have been manifested clinically as hair color. If the patient hair color was not recorded in the medical chart, patients cannot be matched on this variable in retrospect. It is possible that despite matching the patients for the other characteristics, the two groups continue to differ markedly in (unmeasured) hair color and the related key genetic factor. Although the comparison appears fair based on the measured and matched characteristics, an unfair comparison is occurring.
In contrast, randomization assorts patients on all characteristics, measured and unmeasured. Our ability to measure the success of randomization in creating equal groups depends (by definition) on comparing measured characteristics, but with randomization we can be relatively more assured that the assortment of measured characteristics reflects the assortment of unmeasured characteristics. Even with randomization, equal distribution of characteristics between the two groups is not assured. Sometimes, when randomization fails to achieve two groups with similar initial features, researchers then perform statistical “matching” in an attempt to correct for these baseline differences. However, such efforts are no guarantee that the compared groups are indeed similar, as described earlier.
When randomization is not performed, whether the study design is prospective or retrospective, the resulting nonrandom assortment of patients to two treatment strategies creates selection bias. Although the term selection bias sounds nefarious, as if researchers are trying to skew patient treatment in a particular way to influence outcomes, it may be an unconscious practice or a conscious but well-meaning decision. For example, in nonrandomized studies of interventional radiologic techniques, patients may be selected for treatment based on poor clinical status because clinicians or researchers may feel that these patients would benefit most from aggressive therapy. Clinicians and researchers may feel that less-sick patients may not require invasive therapy to do well. The sickest patients of all may not be selected for interventional radiologic therapies because clinicians and researchers may feel that these patients are too unstable or have no hope of survival. If the patients receiving image-guided therapy are subsequently compared to those who did not receive such therapies, outcome differences may be related more to the initial characteristics of the patients than to the therapy used. Sometimes this type of selection bias is evident based on the reported data, but even when such differences are not apparent, selection bias should be suspected whenever randomization is not used.
Some disease processes and injury patterns are not readily subject to prospective study or randomization, often because of the rarity of the condition in question or because the diagnosis is evident only in retrospect. For example, an adequately powered prospective randomized study of an intervention for bowel ischemia would require enrollment of a large population, infeasible at a single medical center. For such conditions, retrospective case-control studies are common. Matching of cases (patients with the condition treated with an intervention of interest) with appropriate control groups (patients with the condition treated with a different standard intervention) to ensure that the groups are as similar as possible at outset is critical, as described earlier. Unfortunately, even with stringent attempts at matching cases and controls, the possibility of unmeasured differences between groups remains, potentially confounding the study results. Although case-control studies sometimes provide the best feasible evidence, we must remain dubious of their results for this reason. A case-control study with statistical matching is not a true substitute for a randomized, controlled trial, no matter how ardently authors may try to convince us.
Let’s consider this in more detail. In some cases, randomization is not performed, and the intervention group is composed of an observed cohort of patients undergoing the therapy in question. A control group is constructed using historical (retrospective) data or contemporaneous patients in another location who are presumed to be similar. Although such studies often are the only source of information about the outcomes of an intervention, they are subject to many biases. For historical controls, unmeasured differences in the types of patients presenting, or in the types of other treatments (other than the measured intervention), may be responsible for outcome differences. For example, if the survival in a modern group of trauma patients treated with a new intervention for splenic trauma is compared with a historical group and found to be superior, it is possible that patients in the past were more severely injured (e.g., because of differences in speed limits or airbag design). Thus the apparent association of improved survival with the new intervention may not reflect causation. Moreover, other aspects of care may be different today from in the past. For example, it is possible that deep vein thrombosis (DVT) and pulmonary embolism (PE) prophylaxis practices were less effective in the past than today. If PE was a major cause of death in trauma patients in the past, mortality rates in the current group might be lower, due not to the study intervention (e. g., splenic embolization) but to behind-the-scenes improvements in other prophylactic measures.
When a contemporaneous but nonrandomized control group is used, differences in patient severity and other treatments, rather than the study intervention, may be responsible for outcomes. For example, imagine that a new intervention is available but only on weekdays during business hours. Researchers may compare patients receiving the treatment with those not receiving it, using the patient’s time of arrival as a “pseudorandomization.” Unfortunately, other factors may be associated nonrandomly with time of arrival and thus with treatment. For example, patients arriving late at night on weekends may be more likely to be intoxicated, and hospital staffing may be different at these times, translating into differences in the quality of care. If an outcome difference is noted between those receiving and those not receiving the therapy, it cannot be assumed that the outcome difference is a consequence of the therapy. As described earlier, researchers often attempt to “correct” statistically for the differences in disease or injury severity, but statistical measures cannot fully account for all potential differences. Beware of such study designs when evaluating the strength of evidence for medical interventions.
Although the limitations described earlier may appear unlikely to influence results of observational studies in important ways, such limitations have famously misled the medical community in the past, with potential harm to patients. In the 1990s, observational studies without randomization demonstrated rates of adverse cardiovascular events that were lower in patients who received estrogen replacement therapy than in those who did not—an association that was widely assumed to be causative. This in turn led to national guidelines recommending the broad implementation of estrogen supplementation in perimenopausal and postmenopausal women, before the completion of randomized, controlled trials to confirm a beneficial causative effect of estrogen therapy. When these randomized, controlled trials (heart and estrogen/progestin replacement studies HERS and HERS II) revealed no reduction in cardiovascular deaths from estrogen use and an increased risk for thromboembolic disease, routine estrogen replacement was largely abandoned.
In cardiology research, observations and theoretical considerations suggested a potential benefit to “facilitated” percutaneous coronary intervention (PCI). This technique uses full- or partial-dose systemic thrombolytic therapy before PCI, which is simply a specialized form of interventional radiologic techniques applied to the coronary arteries. It “made sense” that using thrombolysis (known to have a benefit) in addition to PCI (known to have a benefit) would result in a bigger combined benefit. However, the randomized, controlled Assessment of the Safety and Efficacy of a New Treatment Strategy 4 with PCI showed doubled mortality, increased stroke, and more recurrence of cardiac ischemia from this approach. Experience shows that observational studies without appropriate randomized control groups can lead to mistaken beliefs in the benefit of medical interventions, sometimes with serious patient outcomes resulting.
Blinding
The measures described earlier assist in ensuring a fair “starting line” for the “race,” which is essential to the fair measurement of study outcomes. Blinding techniques are essential to the remainder of the conduct of the study—the equivalent of ensuring a fair referee in a race. Blinding helps ensure that, other than the intervention in question, other treatments of the two groups are similar. In addition, blinding increases the chance that the measurement of outcomes is not influenced by researcher or subject biases about the treatment. Without blinding, even the most honest researcher may be predisposed to see greater benefit in patients receiving a therapy. Without blinding, patients may feel better because of placebo effects of treatment. Even with blinding, this is possible, but the effect should be equal in both the treatment and control group, since patients will not know to which group they are assigned.
Study Populations
Study populations should be representative of the type of patient to which the therapy in question would be applied in clinical practice. Many published studies of interventional radiologic therapies are not representative of the typical emergency department patient but, rather, reflect highly spectrum–biased populations, sometimes including unusually sick patients. Spectrum bias is discussed in more detail in Chapter 15 . Although spectrum bias may not invalidate the study findings if the therapy were applied to similar patients, it does greatly limit the applicability to a more general and diverse patient population—reducing the external validity of the study. Factors that suggest spectrum bias include nonconsecutive patient enrollment, the enrollment of patients after application of many exclusion criteria, and the use of non–emergency department patients, such as surgical intensive care unit patients. For emergency physicians practicing in community hospitals, studies published on tertiary-care medical center patients may be relatively spectrum-biased, and more relevant studies would be those that include both referral centers and community hospital patients.
Publication Bias
A pervasive problem in studies of therapy, as well as diagnosis, is the issue of publication bias—the possibility that studies with negative outcomes may fail to be published. Attempts to limit this include the recent development of study registries, which require that all clinical trials be listed publicly before study initiation (e.g., ClinicalTrials.gov ). Most large journals now require study registration for publication of prospective trials and also attempt to obligate publication of negative results, but this does not solve the potential problem of publication bias in retrospective studies, which form the bulk of evidence for many interventional radiologic therapies. Case series may be particularly subject to publication bias with resulting distortion of the apparent effect of treatments. Authors may be more likely to submit series of “successful” cases for publication, and nonconsecutive case series may systematically omit cases with bad outcomes. Publication bias is thought to lead to major overestimation of treatment efficacy.
Classes of Evidence and Clinical Recommendations
Professional societies such as the American College of Emergency Physicians publish guidelines for practicing physicians, reporting the class of medical evidence summarily and incorporating many of these and other methodologic factors. Clinical recommendations are usually linked to the class of evidence, with level A recommendations based on class I evidence, level B recommendations based on class II evidence, and level C recommendations based on class III evidence (see Table 16-2 ). Class I evidence provides a high level of certainty that the outcomes reported represent the true effects of interventions. Class I evidence usually comes from large prospective trials with adequate statistical power, representative populations of patients, appropriate randomization and blinding, and other measures to limit bias. Metaanalyses are another source of class I evidence. Class II evidence usually comes from weaker studies. These studies may be limited in one or more ways—for example, by limited study size and statistical power; by use of nonrepresentative study populations; by problems of selection bias, such as lack of randomization; or by problems of comparison groups, such as use of historical controls. Despite these limits, the weight of class II evidence may lead the professional organization to make a guarded clinical recommendation in the absence of stronger data. Future studies can specifically target the limitations of these studies, providing class I evidence. Class III evidence is the weakest scientific evidence, often based on single cases, very small case series, highly selection biased samples, nonrandomized studies, and studies with no comparison group. Although such studies provide the starting point for future research, they should be considered as the source of research hypotheses, not the source of precise and unbiased data to guide clinical decisions. Class III evidence, including the eyewitness accounts of interventional radiologists observing dramatic improvements in patient clinical status following treatment, lack many of the key requirements for proof of causation, including a control group, randomization, blinding, and statistical power. Publication bias also likely plays a major role in favoring publication of favorable outcomes in case reports because clinicians may be less likely to report procedural complications and failures.
With this background, we consider some major applications of image-guided procedures. We begin with angioplasty for myocardial infarction because it is common, familiar to emergency physicians, and supported by rigorous research data, forming a strong standard against which we can compare other interventional radiologic techniques. Following this discussion, we take a head-to-foot approach to additional interventions ( Table 16-3 ).
| Emergency Medical Condition | Procedure | Image Guidance | Class of Evidence |
|---|---|---|---|
| Cerebral aneurysm | Endovascular coiling (embolization) to exclude aneurysm from circulation | Fluoroscopy (angiography) | I—Randomized controlled trial showed better outcomes than surgical clipping |
| Ischemic stroke | Clot retriever or intraarterial thrombolysis | Fluoroscopy (angiography) | II/III—Multi-MERCI trial showed feasibility but poor outcomes |
| Acute myocardial infarction | Cardiac catheterization for PCIs; angioplasty and stenting | Fluoroscopy (angiography) | I—Standard of care based on multitude of trials |
| Massive pulmonary embolism | Pulmonary angiography and thrombectomy, intraarterial thrombolysis, or both | Fluoroscopy (angiography) | III—Heroic measure |
| Lower gastrointestinal hemorrhage | Embolization of mesenteric arterial supply | Fluoroscopy (angiography) | III—Commonly used for patients with ongoing blood loss; preferred option before resorting to surgery |
| Traumatic injuries to solid abdominal organs and pelvis with arterial bleeding | Embolization of splenic, hepatic, and pelvic arteries | Fluoroscopy (angiography) | II—Recommended therapy for stable patients with evidence of ongoing hemorrhage; associated with dramatic reduction in frequency of laparotomy and splenectomy |
| Intraabdominal fluid collections or abscesses | Abscess drainage with catheter | CT or ultrasound | II/III—Common method for treating intra-abdominal abscesses, including appendiceal, diverticular, tubo-ovarian, and liver; avoids laparotomy in some cases |
| Massive pulmonary embolism or DVT with contraindication to anticoagulation | IVC filters for PE or DVT; goal is prevention of further embolization of DVT to lung | Fluoroscopy (angiography) | II—Poor evidence of benefit, but often used in patients with transient thromboembolic risk and contraindications to anticoagulation, such as acute trauma with hemorrhage |
| Aortic aneurysm, intact or leaking; aortic dissection or trauma | Aortic endovascular stenting for abdominal aortic aneurysm (AAA), aortic dissection, and aortic trauma | Fluoroscopy (angiography) | I—Randomized controlled trial showed equivalent long-term outcomes to open repair but higher costs; some class II evidence for use in AAA rupture |
| Intravascular foreign body | Foreign body retrieval | Fluoroscopy (angiography) | III—Standard method; far less invasive than vascular surgery, but no systematic evidence |
Angiographically Guided Angioplasty and Stenting of Coronary Arteries for Treatment of ST Segment Elevation Myocardial Infarction
Coronary angiography with the potential for coronary artery intervention such as stenting has become the standard of care for treatment of acute ST elevation myocardial infarction (STEMI). The procedure is usually performed by a cardiologist in the United States, although the instruments and methods are similar to those used by radiologists in other angiographic procedures. A guidewire is introduced into the femoral artery and passed retrograde through the aorta to the coronary arteries. Catheters and stents can be directed to the coronary arteries over the guidewire. The instruments and vessel lumen are visualized using fluoroscopy and a technique called digital subtraction angiography. In this technique, images of the bones and soft tissues (sometimes called a “mask”) acquired before the administration of arterial iodinated contrast agents are digitally removed from the images acquired after contrast administration. This results in images of vascular structures without obstruction by superimposed radiodense anatomy.
Introduced in 1976, percutaneous transluminal coronary angioplasty (PTCA) for STEMI saw broad clinical adoption by the early 1980s. Large randomized, controlled, multinational trials in the mid-1990s confirmed the benefits of the procedure. The Global Use of Strategies to Open Occluded Coronary Arteries IIb trial randomized 1138 STEMI patients to PTCA or thrombolytic therapy with recombinant tissue plasminogen activator (t-PA) within 12 hours of STEMI and demonstrated a statistically significant reduction in 30-day mortality in patients treated with PTCA (5.7%) compared to patients treated with t-PA (7.0%). Six-month outcomes of death, nonfatal reinfarction, and nonfatal disabling stroke were not significantly different between the two groups. Both PTCA and t-PA outcomes were improved compared with the historical mortality among STEMI patients receiving aspirin, shown to be 9.4% in the second International Study of Infarct Survival (ISIS-2) study. This, in turn, was a substantial improvement in comparison with patients in ISIS-2 randomized to no aspirin, who had a mortality of 11.8%. Cucherat et al. performed a Cochrane meta-analysis in which only randomized, controlled trials with no confounding were included. Ten trials totaling 2573 patients showed early primary angioplasty to be superior to systemic intravenous (IV) thrombolysis in experienced centers for short-term mortality. Due to class I evidence for its use, PTCA has become the standard of care for STEMI when available within 3 hours.
Image-Guided Therapy for Ischemic Stroke
Ischemic stroke remains a morbid condition, with limited evidence for treatment safety and efficacy. Controversy has continued around the use of systemic thrombolysis because of conflicting interpretation of data, methodologic concerns, and the possibility that t-PA might harm patients through increased intracranial hemorrhage. The National Institute of Neurological Disorders and Stroke (NINDS) trial randomized patients to IV t-PA or placebo and demonstrated a 30% increase in the likelihood of having no or minimal neurologic deficit at 3-month follow-up in patients treated with t-PA, although the results have been contested because of concerns that the two treatment groups differed in stroke severity before treatment, confounding results. Katzan et al. reported the Cleveland community experience with systemic IV t-PA. In-hospital mortality was 15.7% among patients receiving t-PA, compared with only 5.1% among those not receiving t-PA. 15.7% of patients receiving t-PA also had symptomatic intracranial hemorrhage, including six fatalities. Of the treated patients, 50% suffered protocol violations. This study is a retrospective review; patients were not randomized to receive t-PA, so it is possible that differences in mortality between treated and untreated patients resulted from their preexisting clinical status, not from their treatment. Nonetheless, studies such as this suggest that the positive risk–benefit ratio reported in closely controlled clinical trials such as NINDS might not be achieved if t-PA were widely adopted in the community.
Even if IV t-PA is assumed to be beneficial, systemic thrombolysis remains a possibility for a small number of patients because of tight time constraints from symptom onset until treatment initiation (3 hours based on the NINDS trial and as much as 4.5 hours based on data from the third European Cooperative Acute Stroke Study ) and numerous contraindications. Only about 15% of patients arrive within 3 hours after symptom onset. Schumacher et al. analyzed the National Inpatient Sample and found that 70% of hospitals did not use t-PA. Only approximately 1% of 366,194 ischemic stroke patients received t-PA, an average of only about 3 patients annually per hospital using t-PA.
With questions about benefit or harm of systemic t-PA therapy and limited populations meeting treatment criteria, interest has focused on catheter-based treatment techniques that might be more effective in relieving neurologic symptoms, extend the treatment time window and thus allow therapy for more patients, and have fewer contraindications because of a lower dose of t-PA and lower bleeding risks. These studies examine mechanical clot retrieval, intraarterial t-PA administration, and intracranial artery stenting, all performed under fluoroscopic angiographic guidance. Let’s examine some relevant studies and assess the quality of evidence.
Smith et al. reported the results of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial. This study enrolled 141 patients with ischemic stroke who were ineligible for t-PA and performed mechanical embolectomy within 8 hours of symptom onset using an intraarterial device, the MERCI Retriever. This prospective multicenter study was not randomized and therefore used a historical control group, with the methodologic limitations described earlier in this chapter. Vessel recanalization was achieved by 46% of enrolled patients, compared with only 18% in the historical control group. Good neurologic outcomes occurred in 46% of patients who achieved recanalization, compared with only 10% of those who did not. Mortality was lower in patients who achieved recanalization (32%) compared with those who did not (54%). Significant complications of the procedure occurred in 7.1%, and symptomatic intracranial hemorrhage occurred in 7.8%. Although the authors concluded that the device was useful within 8 hours of symptom onset in patients ineligible for t-PA, the study cannot prove that catheter-based mechanical embolectomy improves outcomes, given the absence of randomization or a contemporaneous control group.
Smith also reported the early results of the Multi-MERCI trial, which enrolled patients who had received systemic IV t-PA but failed to recanalize, as well as patients who were ineligible to receive t-PA. The prospective multicenter trial had no randomization and treated all patients within 8 hours of symptom onset with mechanical catheter-based clot retrieval. The trial enrolled 111 patients, and 27% had received IV t-PA before enrollment. Vessel recanalization was achieved by 54% of patients with the mechanical device alone, and an additional 15% achieved this when further treatment with intraarterial t-PA was performed. Significant procedural complications occurred in 4.5% of patients. Symptomatic intracranial hemorrhage occurred in 6.7% of those who had been treated with IV t-PA before mechanical embolectomy and in 9.9% of those who had not been so treated. Again, this study cannot prove improved neurologic outcomes without the use of randomization and in the absence of a control group.
Kim et al. retrospectively reviewed 24 patients treated with the MERCI Retriever outside of the MERCI trial. Of these, 9 patients were ineligible for the MERCI trial because 4 patients had received prior IV t-PA, 1 patient had received IV t-PA and was younger than 18 years, and 4 patients had exceeded 8 hours since symptom onset. Recanalization was achieved in 63%, using a combination of mechanical clot retrieval and (in some cases) intraarterial t-PA and abciximab (a glycoprotein IIb–IIIa inhibitor of platelet activity). Asymptomatic hemorrhage occurred in 38%, and symptomatic hemorrhage occurred in 8%. In-hospital mortality was 17%, 90-day mortality was 29%, and good 90-day neurologic outcomes occurred in 25%. Although the authors concluded that mechanical embolectomy is an effective means of achieving revascularization for ischemic stroke, this study cannot prove any outcome benefit to patients without randomization or use of a control group. We cannot even know whether patients might have been harmed by the device because outcomes for a control group are not available.
Layton et al. reported a case of left internal carotid artery occlusion treated 8 hours after symptom onset using the MERCI Retriever, with significant improvement in neurologic symptoms. The authors concluded that “the final infarct area, as demonstrated on magnetic resonance imaging, was probably much smaller than it would have been if the vessel had not been recanalized.” Such statements, while reflecting the optimism of researchers and interventional radiologists that they may improve patient outcomes, do not constitute proof of efficacy and reveal the bias inherent in such reports. Randomized, controlled trials, with blinded assessment of neurologic outcomes by independent observers, are needed.
Choi et al. examined the frequency of endovascular recanalization therapies using the National Inpatient Sample from 1999 to 2002 and found that 0.17% of ischemic strokes were treated with these therapies. The authors also examined outcomes in patients treated with these therapies at Columbia University Medical Center from 2001 to 2004. From the Columbia University sample, 32% of patients treated with endovascular therapies achieved modified Rankin scales of 0 to 2 (indicating slight disability or better, see Table 16-4 ), and 29% died—one fifth from intracranial hemorrhage. In the national sample, 15% died and 50% were discharged to home or a rehabilitation facility. Despite the lack of a control group for comparison, the authors concluded that the technique is safe and effective.
| Modified Rankin Score | Description |
|---|---|
| 0 | No symptoms at all |
| 1 | No significant disability despite symptoms; able to carry out all usual duties and activities |
| 2 | Slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance |
| 3 | Moderate disability; requiring some help, but able to walk without assistance |
| 4 | Moderately severe disability; unable to walk without assistance and unable to attend to own bodily needs without assistance |
| 5 | Severe disability; bedridden, incontinent and requiring constant nursing care and attention |
| 6 | Dead |
Shaltoni et al. retrospectively assessed data from a prospectively collected database and reported outcomes in 69 patients treated with a variety of intraarterial thrombolytic agents via a catheter after failure to recanalize with IV t-PA. Symptomatic intracranial hemorrhage occurred in 5.8%, including three fatal cases. Recanalization was reported in 72.5%, and a favorable neurologic outcome (defined as discharge to home or inpatient rehabilitation) in 55%. Like the prior studies, this case series suggests possible safety and efficacy but lacks randomization, a consistent therapeutic intervention, or any control group.
Sauvageau et al. retrospectively reviewed 10 cases in which middle cerebral artery stenting was performed after failure of recanalization with the MERCI Retriever and (in some cases) intraarterial t-PA. In this review, 9 of 10 patients achieved recanalization, although the rate of complications was high: it included 6 cases of intracranial hematoma or subarachnoid hemorrhage, 1 case of extradural perforation with arteriovenous fistula formation, and 4 deaths. This small case series without randomization or a control group raises the possibility of this technique but does not prove any benefit.
Gonzalez et al. retrospectively reviewed a series of nine patients with ischemic stroke treated with catheter-based mechanical thrombectomy with a microsnare and low-dose intraarterial t-PA or angioplasty. They report 77.8% clot removal. At 3 months, two patients had a normal modified Rankin scale, three patients had significant neurologic impairment (modified Rankin scale of 3 to 4), and two patients had died. The authors concluded that the device is safe, but larger randomized, controlled trials would be required to prove benefit.
Smith et al. reported final results of the Multi-MERCI trial, including patients treated with a newer generation MERCI Retriever (the L5 Retriever). Although higher rates of recanalization, lower mortality, and better neurologic outcomes were associated with the newer device compared with the older device, these did not achieve statistical significance. Moreover, because patients were not randomized to one device or the other and no third control arm existed (untreated with either device), we cannot conclude that either device is superior to no treatment.
Class II evidence including nonrandomized trials and case series suggests that catheter-based endovascular therapies, including mechanical clot retrieval and intraarterial thrombolysis, are feasible. However, in the absence of large randomized, controlled trials, it is premature to conclude that neurologic outcomes and mortality are improved by the use of any of these techniques. Suggested indications for interventional neuroradiologic therapies are shown in Box 16-1 .
- •
Symptom onset > 3-4.5 hours prior, precluding systemic thrombolysis
- •
Other contraindications to thrombolytic therapy, making mechanical clot extraction preferable or the only alternative
- •
Failure to recanalize the thrombosed vessel after systemic thrombolytic therapy
- •
Posterior circulation stroke or middle cerebral artery thrombosis
Angiographic Embolization for Aneurismal Subarachnoid Hemorrhage
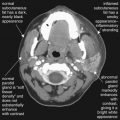
Catheter-based therapy with endovascular embolization has become the accepted method of treatment for most aneurismal subarachnoid hemorrhage. Before the introduction of this technique, craniotomy and surgical clipping of the aneurysm neck was required, a highly invasive procedure. To perform endovascular embolization, an arterial catheter is inserted and advanced into the internal carotid artery and then to the location of the aneurysm, which is usually positioned along the circle of Willis or arterial branches emanating from this vascular ring. The position of the catheter is identified by fluoroscopy using digital subtraction of bony structures as described earlier in the section on coronary artery interventions. Intraarterial iodinated contrast is injected to reveal the intracranial vasculature, including the aneurysm sac. A thin metal wire is advanced into the aneurysm and coiled within the sac, slowing blood flow and leading to internal thrombosis of the aneurysm ( Figure 16-1 ). This excludes the aneurysm from the arterial circulation, dramatically decreasing the risk for rupture.

Molyneux et al. conducted a prospective controlled trial of patients with subarachnoid hemorrhage from ruptured intracranial aneurysms, randomized to treatment with either endovascular embolization (“coiling”) or neurosurgical clipping. The International Subarachnoid Aneurysm Trial (ISAT) was planned to include 2143 patients but was stopped early after a planned interim analysis showed better outcomes in patients undergoing endovascular therapy. At 1-year follow-up, 190 of 801 (23.7%) patients randomized to endovascular therapy were dependent or dead, compared with 243 of 793 (30.6%) treated surgically ( p = 0.0019). The relative and absolute risk reductions for dependency or death using endovascular therapy were 22.6% (95% CI = 8.9-34.2) and 6.9% (95% CI = 2.5-11.3), respectively. Re-bleeding risks at 1 year were minimal in both groups. In a second publication, the same authors reported the survival advantage of endovascular coiling to persist up to 7 years.
Van der Schaaf et al. performed a Cochrane meta-analysis, identifying three randomized trials comparing endovascular embolization (coiling) with surgical clipping for treatment of aneurismal subarachnoid hemorrhage. One of the included trials was ISAT, discussed earlier, which dominates the overall effect because it includes by far the most enrolled patients. The trials included 2272 patients, most with anterior cerebral circulation aneurysms and in good clinical condition before their procedure. At 1-year follow-up, poor neurologic outcomes were less common in patients randomized to coiling, with a relative risk of 0.76 (95% CI = 0.67-0.88). The absolute risk reduction for bad neurologic outcomes was 7% (95% CI = 4%-11%) for patients treated with coiling compared with patients treated surgically. For patients with a posterior circulation aneurysm, the relative risk for poor neurologic outcome was 0.41 (95% CI = 0.19-0.92) with an absolute decrease of 27% (95% CI = 6%-48%).
As a consequence of these trials, which provide class I evidence of benefit from endovascular coiling of cerebral aneurysms with subarachnoid hemorrhage, endovascular therapy has become the standard approach. Not all aneurysms are amenable to endovascular coiling. The neck of the aneurysm must be relatively narrow to allow the coil to be trapped within the aneurysm sac, and the aneurysm cannot include the origins of branch vessels; otherwise, they could be thrombosed by the coiling. These features of the aneurysm sac are often evaluated today by CT angiography (CTA) with three-dimensional reconstruction, before conventional angiography. For aneurysms 5 mm or larger, CTA is approximately 94% sensitive and magnetic resonance angiography is approximately 86% sensitive.
Interventional Radiologic Therapies for Pulmonary Embolism
Neither standard therapy with systemic anticoagulation nor aggressive therapy with systemic thrombolysis eliminates the morbidity and mortality of PE, leading to interest in image-guided interventions. Naess et al. found that the 30-day case-fatality rate in PE was 9.7% using population-based data from 1995 to 2001. Nijkeuter et al. reported 3-month mortality of 8.2% in patients treated for PE with anticoagulation. Thabut et al. performed a metaanalysis of nine randomized, controlled trials (461 patients) comparing heparin with thrombolysis. The studies varied considerably in methodology: eight used systemic drug administration, and only one used intrapulmonary drug administration. Thrombolysis had no statistically significant effect on mortality. The relative risk for mortality was 0.63 for patients treated with thrombolysis, with 95% CIs from 0.32 to 1.23. When 95% CIs for relative risk span the value 1.0, this indicates a lack of statistical significance because the true relative risk may be decreased (<1) or increased (>1). However, because the mean value was 0.63, which would suggest a potential mortality risk reduction of more than 30%, one interpretation is that a very large trial might yet prove a statistically significant mortality reduction. Metaanalyses such as this demonstrate the difficulty of attempting to determine the potential benefit or harm of a therapy from multiple small trials with varying methodology—a large multicenter trial following a standardized protocol is needed. Similar problems plague the existing literature on image-guided, catheter-based therapies, discussed later.
Konstantinides et al. conducted just such a trial in 256 hemodynamically stable patients with submassive PE, characterized by pulmonary hypertension or right ventricular dysfunction without systemic arterial hypotension or shock. They randomized 118 patients to heparin plus systemic IV t-PA and 138 patients to heparin plus placebo. The trial showed a statistically significant decreased requirement for escalated therapy (catecholamine infusion, secondary thrombolysis, endotracheal intubation, cardiopulmonary resuscitation, or emergency surgical or catheter thrombectomy) in patients treated with systemic t-PA (10.2%) compared with patients receiving placebo (24.6%). However, no statistical difference in mortality was observed. Mortality was 3.4% in patients treated with heparin and t-PA and 2.2% in those receiving heparin and placebo ( p = 0.71).
Mortality from PE may not tell the complete tale; significant morbidity from PE may occur, with secondary pulmonary hypertension developing in as many as 8.8% and symptomatic pulmonary hypertension in 4.4%. Tapson and Humbert estimated chronic thromboembolic pulmonary hypertension to be 1% 6 months after PE, 3.1% 1 year after PE, and 3.8% 2 years after PE. Piovella et al. concluded that 1% to 4% of patients with PE develop chronic thromboembolic pulmonary hypertension.
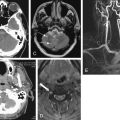
Given the high mortality and morbidity of PE, numerous investigators have attempted catheter-based therapies for PE, including thrombectomy with mechanical clot fragmentation and aspiration, as well as intrapulmonary artery administration of thrombolytic agents ( Figure 16-2 ). Goals of these therapies include reducing acute mortality and long-term sequelae of pulmonary hypertension. Large obstructing pulmonary emboli might create less hemodynamic compromise when fragmented and either mechanically removed or allowed to embolize distally. Clot fragmentation might increase the exposed surface area of clot, improving the action of thrombolytic agents when these are used with mechanical clot disruption. An additional potential benefit of catheter-based therapies is that mechanical clot disruption and removal can be used in patients with contraindications to systemic anticoagulation or thrombolysis. Tumor emboli (such as tumor fragments from invasive renal cell carcinomas) that do not benefit from anticoagulation or thrombolysis can be treated using image-guided, catheter-based therapies. Let’s consider the evidence for these therapeutic approaches, keeping in mind that strong evidence would require consecutive subject enrollment, adequate study size to provide statistical power, use of a concurrent control group, randomization to treatment arm, and blinded measurement of clinically important patient outcomes. As we demonstrate, the existing studies fulfill few of these criteria, and even the inclusion criteria and indications for image-guided therapies are not uniform.

Tibbutt et al. randomized 30 patients to intrapulmonary artery infusion of either heparin or streptokinase. The study lacked blinding and did not follow an intention-to-treat analysis as is recommended today. Instead, 7 patients who failed to complete the protocol were excluded from analysis, leaving only 12 patients in the heparin group and 11 in the streptokinase group. The angiographic appearance of clot and pulmonary arterial pressures were improved to a greater extent in the streptokinase group initially and at 6 months, but meaningful clinical outcomes were not measured. The small study size and exclusion of nearly one third of patients from analysis limits the value of the study. Streptokinase is also no longer the thrombolytic agent typically in use today.
Verstraete et al. compared systemic IV t-PA to intrapulmonary artery administration (performed during fluoroscopic pulmonary angiography) in 34 patients with massive PE. Both therapies improved pulmonary artery pressures and angiographically measured severity of PE, with no apparent advantage of intrapulmonary artery therapy. However, this study was not powered to determine superiority of one approach over the other and did not measure important clinical endpoints. In addition, techniques and devices for pulmonary angiography have evolved subsequently, leading some interventional radiologists to discount this study.
Timsit et al. reported catheter pulmonary embolectomy in 18 patients with massive PE, with 72% survival. Greenfield et al. reported a series of 46 patients with massive PE undergoing vacuum extraction of clot by catheter between 1970 and 1992, with a 30-day survival of 70%. Tajima et al. described three cases of superselective t-PA infusion through a pulmonary artery catheter, with immediate angiographic improvement. However, no clinical outcomes or comparison group were reported. Uflacker et al. described five patients with massive PE treated with a catheter device that pulverized thrombus using a vortex. One patient died shortly after the procedure; the four remaining patients survived to discharge. Stock et al. reported five patients with massive PE treated by thrombus fragmentation and intrapulmonary artery injection of t-PA. All five showed angiographic improvement and improved pulmonary artery pressures, but two required transfusion for retroperitoneal hematomas.
Schmitz-Rode et al. described mechanical fragmentation of massive pulmonary emboli in 10 patients using a rotating pigtail catheter and supplemented in 8 patients with thrombolysis. Clot fragmentation was successful in 7 cases. Improvements in shock index were observed, and 48-hour pulmonary pressure measurements also decreased significantly. Despite this, the mortality was 20%. The authors reported that no procedural complications occurred, though safety cannot be established by such a small study. This study cannot prove a clinical benefit to patients, given its small size and lack of control group. Nonetheless, it demonstrated the feasibility of mechanical fragmentation.
Rocek et al. reported a single case of mechanical thrombectomy using a percutaneous catheter. Murphy et al. reported four patients with massive PE treated with percutaneous catheter clot fragmentation using standard angiography catheters and guidewires, followed by local t-PA administration. All four survived to hospital discharge and had improved pulmonary angiography findings.
Fava et al. described 11 patients with massive PE undergoing mechanical thrombectomy with a catheter device. Only 1 patient died during the procedure—the authors concluded the death was because of PE, not procedural complications. The other 10 patients had improved pulmonary artery pressures and arterial oxygen partial pressures and survived to discharge.
Schmitz-Rode et al. reported 20 patients with massive PE treated by mechanical fragmentation with a rotating pigtail catheter, some with additional thrombolysis. Shock index and pulmonary artery pressures improved, and mortality was 20%. It is unclear whether the patient group reported included some of the same patients described in the 1998 publication by the same group (described earlier).
De Gregorio et al. treated 59 patients with massive PE using mechanical clot fragmentation and intrapulmonary thrombolysis. The authors reported 6% mortality, with clinical improvements and improved pulmonary artery pressures in all survivors at 3 to 6 months.
Zeni et al. described fragmentation and aspiration of massive pulmonary emboli using high-velocity saline jets from a specialized catheter. They treated 17 patients, and 10 received additional thrombolytic therapy via the catheter. Angiographic improvement and initial improvement in dyspnea and oxygen saturation were seen in 16 of 17 patients. Although 2 patients died, the remaining 15 survived to hospital discharge, though long-term follow-up was not reported. Again, no control group was used in this study, preventing comparative assessment of the effect of therapy.
Reekers et al. described eight patients treated with both mechanical catheter thrombectomy and systemic thrombolysis. Normalization of partial pressure of oxygen values occurred in all patients, with minimal change in pulmonary arterial pressures. The authors were only moderately successful in removing clot using their technique, with a mean of 50% removed. The authors noted immediate symptomatic improvement in all patients; however, one patient died shortly after the procedure, reportedly from heart failure. Despite this, the authors asserted no procedural complications. Small studies such as this highlight the need for blinding and a control group in establishing not only benefit but also harm. It is possible that the procedure contributed to the death of one patient, but the unblinded authors did not draw this conclusion. Without randomization or a control group, we cannot establish any causal relationships or estimate the benefit or injury related to this technique.
Tajima et al. reported treatment of 25 patients with massive PE with mechanical fragmentation using a rotating pigtail catheter, with local thrombolytic therapy and clot aspiration, followed by systemic thrombolysis. All patients survived the procedure, with improvements in angiographic appearance and pulmonary artery pressures. However, long-term clinical outcomes were not reported. The authors attributed two deaths before discharge to ovarian and lung cancers—not to PE or procedural complications. Several problems exist with attempts to interpret this data. The authors did not describe their inclusion criteria, so the clinical status of the patients undergoing this therapy is uncertain. No control group, randomization, or blinding was present, so the potential benefit or harm of the intervention cannot be determined. Tajima et al. also reported 15 patients with massive PE treated by manual aspiration of clot through a standard large lumen catheter designed for PTCA. All patients survived the procedure.
Despite the multitude of small studies describing clinical experience with a variety of image-guided, catheter-based therapies, we cannot draw any firm conclusions about the benefit, harm, or indications for these interventions because of the lack of appropriate methodology. A large randomized, controlled trial with blinding is needed to establish evidence of benefit. In the absence of such evidence, the 2004 guidelines of the Seventh American College of Chest Physicians Conference on Antithrombotic and Thrombolytic Therapy recommended against the use of catheter-based therapies except in patients unable to receive thrombolytic therapy or whose critical status does not allow the 2-hour infusion time for systemic t-PA. Future studies will require more standardized inclusion criteria with stratification of patients with similar mortality risk to allow fair comparisons of outcome. Let’s briefly examine some recent studies on PE prognosis.
Pulmonary Embolism Prognosis: Role of Imaging and Biomarkers
One basic point of disagreement is the definition of massive PE, which is a vital starting point for studies purporting to examine the effect of catheter-based therapies. Some studies characterize massive PE by patient clinical response, including hypotension requiring inotropic or vasopressor support or respiratory failure requiring mechanical ventilation. Others use clot burden determined by imaging criteria, even in the absence of current severe hemodynamic or respiratory compromise. Still others characterize massive PE by biomarker surrogates of heart failure. The tantalizing promise of imaging or biomarker criteria is that these might be shown to predict risk for subsequent deterioration or death in currently stable patients. This in turn might allow selection of patients for invasive therapy while the patients are still able to tolerate the procedure and before such severe clinical deterioration that no intervention would likely improve mortality.
Engelke et al. described a CT scoring system to predict cor pulmonale and short-term survival in massive PE. In a retrospective study of 89 consecutive patients with acute PE diagnosed by CTA, a CT severity score was more predictive of elevated pulmonary artery pressures, cor pulmonale, and death within 30 days than were two severity scores based on conventional angiography.
Ghaye et al. reviewed the literature on CT clot burden scores and reported their limited prognostic value. In a retrospective study of 82 consecutive patients admitted to an intensive care unit for PE and evaluated with CT, the same authors measured a variety of cardiac and great vessel diameters and pulmonary artery clot burden and correlated these with patient outcome. Clot burden measured by CT did not correlate with inpatient mortality, whereas the right ventricle–left ventricle ratio and azygos vein dimensions (both thought to be surrogates of right heart failure) did correlate strongly with survival.
He et al. compared CT measurements and echocardiographic findings of right heart failure. In their retrospective study of 74 consecutive adults with PE diagnosed by non-cardiac-gated CT, right heart dysfunction was diagnosed if the right ventricle appeared dilated on visual inspection (no specific diameter or ratio defined) or if the interventricular septum appeared straightened or bowed into the left ventricle. Somewhat counterintuitively, the authors concluded that CT findings were more sensitive (81% CT, 56% echo) and specific (47% CT, 42% echo) than echocardiography findings in demonstrating right heart dysfunction. The reference standard for right heart dysfunction in this study is questionable: pulmonary vascular obstruction of at least 30% as measured on CT. It is unclear whether this measure should be considered more indicative of right heart dysfunction than echocardiographic findings. The CT findings of right heart failure were not compared with patient outcomes, the more clinically relevant endpoint. In addition, the reported sensitivity and specificity values for CT are likely too low to be clinically useful because one in five cases of right heart dysfunction would be missed and half of CT-diagnosed cases of right heart failure would be false positive.
In a larger study comparing CT findings to clinical outcomes, Araoz et al. retrospectively reviewed CT scans in 1193 patients with CT scans positive for PE and concluded that embolic burden and the ratio of right ventricular to left ventricular diameter were not predictive of short-term mortality. Ventricular septal bowing into the left ventricle was insensitive (around 20%) but specific (87%) for short-term mortality, defined as in-hospital death or death within 30 days of CT.
Ryu et al. retrospectively reviewed 546 consecutive patients with acute PE diagnosed by CTA and found 14 patients (2.6%) with saddle PE, none with preexisting cardiopulmonary disease. Saddle PE had previously been described as a likely marker of PE mortality. The authors noted that none of the patients died during their initial hospitalization and thus concluded that saddle PE may not require aggressive medical management in the absence of comorbid cardiopulmonary disease. However, this conclusion may be limited by several factors. One patient was treated with thrombolytic therapy, and 4 others received inferior vena cava (IVC) filter placement, interventions that may have limited their mortality. Also, 4 patients (29%) died within 1 year. Finally, the authors did not assess for morbidity such as pulmonary hypertension in these patients.
More work remains to be done in identifying CT findings that strongly predict mortality or morbidity from PE and thus might be indications for invasive therapy. Suggested indications are listed in Box 16-2 .