Infections caused by enteroviruses, rabies, adenoviruses, and Nipah and Hanta viruses are discussed. Several studies defined the pattern of MR imaging findings in these disease processes that reflect parenchymal infiltration with inflammatory cells, typically visualized as areas of low attenuation on CT, as well as of low T1 and high T2 signal intensity on MR imaging. Diffusion-weighted MR imaging has been shown to be superior to conventional magnetic resonance imaging for the detection of early signal abnormalities in encephalitis. Focal unilateral hyperperfusion as visualized by SPECT appears to be an indicator of severe inflammation of the brain tissue and was found to be an independent predictor of poor prognosis, whereas clinical outcome variables, CSF, or EEG findings are not.
Over 100 different viruses have been identified as causative agents of central nervous system (CNS) infections. Viruses gain access to the CNS through hematogenous dissemination or, less frequently, along the peripheral nerves, and many have a predilection for the gray matter, whether it is cortical, deep, or intramedullary. Other viruses, such as HIV and CJ virus, prefer the white matter . A number of viral and other infectious agents characteristically lead to specific topographic lesions in the CNS, for instance temporal lobe involvement with herpes simplex virus (HSV) or rhombencephalitis with Listeria monocytogenes . However, there are certainly exceptions to this rule and the “topographic” viruses may lead to nospecific presentation, while other infectious agents and even noninfectious disease processes may simulate characteristic topographic appearances, both clinically and on the imaging studies.
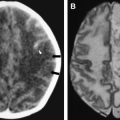
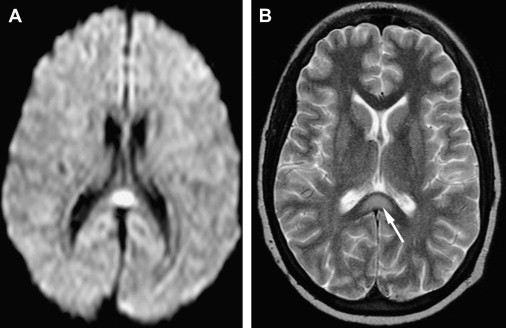
It needs to be mentioned that a number of different studies confirmed that the etiology of encephalitis remains unknown in many cases of this complex syndrome, since probable etiologic agent was established in only 16% to 69% of cases of encephalitis . A majority of the identified agents are viral, and in addition to common herpes simplex (HSV) and Varicella-zoster virus (VZV), enteroviruses and adenoviruses are relatively frequently encountered. Additionally, certain specific anatomic lesions may be present with a number of different viral infections, as well as with other etiologies. For instance, transient lesion of high T2 signal and decreased diffusion in the splenium of the corpus callosum ( Fig. 1 ) have been documented with influenza, adenovirus, and rotavirus encephalitides, but also with Escherichia coli infection, epilepsy, antiepileptic medications, hypoglycemia, osmotic myelinolysis, and other disease processes . In a similar fashion, topographic diagnosis of brainstem encephalitis with swelling and signal abnormality on imaging studies may be caused by a number of different infectious agents .
In this article, infections caused by enteroviruses, rabies, adenoviruses, and Nipah and Hanta viruses are discussed. Several studies defined the pattern of MR imaging findings in these disease processes that reflect parenchymal infiltration with inflammatory cells, typically visualized as areas of low attenuation on CT, as well as of low T1 and high T2 signal intensity on MR imaging. Diffusion-weighted MR imaging has been shown to be superior to conventional magnetic resonance imaging for the detection of early signal abnormalities in encephalitis . Focal unilateral hyperperfusion as visualized by single-photon emission computed tomography (SPECT) appears to be an indicator of severe inflammation of the brain tissue and was found to be an independent predictor of poor prognosis, whereas clinical outcome variables, CSF, or EEG findings are not .
Enteroviruses
Enteroviruses are among the most common etiologic agents responsible for CNS infections in their hosts. They are small linear, single-stranded nonenveloped eicosahedral RNA viruses that belong to Picornaviridae family. The enteroviruses include Coxsackie viruses A and B, poliovirus, echoviruses, and enteroviruses (EV) 68 to 71. They may cause a number of disease processes, including poliomyelitis, meningitis, and encephalitis. Nonpolio enteroviral encephalitis usually presents as a diffuse, generalized encephalitis. Focal cerebral involvement by nonpolioviruses is uncommon, and neuroradiologic studies in these cases are usually normal. Coxsackie virus A16 and EV 71 lead to hand-foot-mouth disease (HFMD); EV 71 to herpangina; EV 70 and Coxsackie virus A24 to hemorrhagic conjunctivitis; poliovirus to poliomyelitis; EV 70, EV 71, and Coxsackie virus A7 and A24 to polio-like paralysis, or radiculomyelitis . Although the poliovirus receptor is produced in a broad range of organs, viral replication and disease are limited to a few sites, including the brain, spinal cord, and alimentary tract. The outcome of infection is determined by a complex interplay between virus and host.
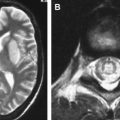
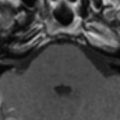
Poliomyelitis caused by poliovirus has almost disappeared in developed countries, thus the reports of MR imaging findings in poliomyelitis are rare, with characteristic cervical spinal cord and brain stem lesions of high T2 signal . The pathologic CNS involvement by enteroviruses is characteristically located in the posterior portion of the medulla oblongata (the dorsal nuclei of the vagus nerve, the medial longitudinal fasciculus, the reticular formation, and the nuclei of the solitary tract), posterior portion of the pons (the nuclei of cranial nerves VI, VII, and IX), central portion of the midbrain (the red nuclei, substantia nigra, the nuclei of cranial nerves III and IV), bilateral dentate nuclei of the cerebellum, and bilateral ventral horns of cervical spinal cord. Why these areas are the predilection sites of CNS involvement of enteroviruses remains unknown . MR imaging studies accordingly show T2 hyperintensity in the posterior aspect of medulla oblongata and pons, in the midbrain, the dentate nuclei of the cerebellum, and spinal cord ( Figs. 2 and 3 ); the thalamus and putamina may rarely be involved. Symmetric bilateral lesions in the dorsal brain stem and ventral horns of the cervical spinal cord are considered characteristic MR findings of enteroviral encephalomyelitis .
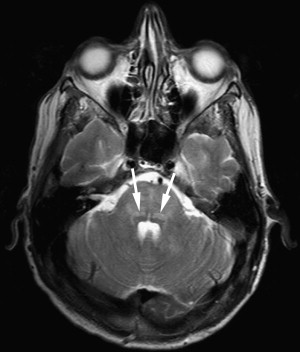
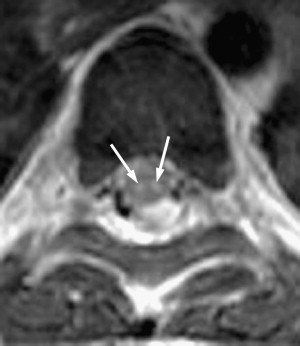
EV 71 affects the same locations of the brain as does poliovirus; a small portion of patients have symptoms of polio-like encephalitis and paralysis. EV 71 infection should be suspected in young children with epidemic HFMD or herpangina complicated by encephalitis or acute flaccid paralysis in the limbs. Epidemic outbreaks of EV 71 occurred in Bulgaria in 1975, in Hungary in 1978, in Malaysia in 1997, and in Taiwan in 1998, primarily affecting young children, with 20% to 40% of CNS morbidity and significant mortality rates . During the acute stage, T2-weighted images revealed hyperintense areas without abnormalities on T1-weighted images, reflecting acute inflammation. Most patients recover well after the appropriate management and the lesions completely disappear within weeks to months. In patients with poor outcomes, follow-up MR imaging months later reveals T1 hypointense lesions with hyperintensity on T2-weighted images, reflecting irreversible tissue destruction in the brainstem and cervical spinal cord. In a small subset of patients with the highest grade of clinical symptoms, in whom brain stem encephalitis is always present, fatal outcomes frequently occur in a very short period of time. Fatal outcomes are a result of acute cardiac failure and/or pulmonary edema, for which neurogenic cardiac damage or neurogenic pulmonary edema have been proposed as the underlying mechanisms , related to the brain stem lesions, especially in the posterior aspect of the medulla oblongata.
EV 71 may also present as radiculomyelitis with unilateral lesions involving the anterior horns of the cervical or thoracic spinal cord and ventral roots . These anterior horn lesions are sometimes accompanied by ventral root contrast enhancement. It seems that this unilateral acute flaccid paralysis (AFP) is most likely reversible. AFP radiculomyelitis is potentially distinguishable from poliomyelitis by the MR characteristics of unilateral cord involvement and root enhancement . Patients with less common bilateral AFP and bilateral anterior horn lesions appear to have a less favorable outcome.
It needs to be stressed that AFP may be caused by a variety of enteroviruses and not just by poliovirus, and that this topographic lesion distribution is even less specific, as Japanese encephalitis and Epstein-Barr viruses may lead to the same clinical presentation and neuroimaging appearance . Also, these lesions have been described with Hopkins syndrome, a poliomyelitis-like illness manifesting flaccid paralysis of an extremity in the recovery stage after an asthmatic attack. A patient with Hopkins syndrome had T2 hyperintensity of the left anterior horn in the cervical spinal cord .
On the other hand, enteroviruses may cause CNS infections with nonspecific imaging appearance or simulate other topographic involvement characteristics for other infectious agents. Rapidly progressive paraplegia with bowel and urinary dysfunction, consistent with clinical diagnosis of acute transverse myelitis, has been described as a rare clinical manifestation of Coxsackie virus (B4 and B5) infection. MR imaging showed diffuse swelling and increased T2 signal in the inferior portion of the spinal cord at T1-L2 level . Bilateral hippocampal lesions on MR imaging were found in another case and suggest that enteroviral infection should be included in the differential diagnosis of acute temporal lobe encephalitis not caused by herpes simplex virus . Recently, cases of enteroviral meningoencephalitis in infants that looked similar to periventricular leukomalacia have been reported. Increased echogenicity was present on cranial ultrasonography in the periventricular white matter, and MRI confirmed mild to severe white matter damage in all cases .
Rabies
Rabies is an almost invariably fatal CNS infection that is transmitted to humans from bites by rabid animals, most commonly dogs. It is one of the most important encephalitides worldwide with at least 40,000 deaths reported annually. If no postexposure vaccination is given, the risk of developing rabies after the bite of a rabid animal is about 50%, depending on the location and severity of the bite . Other possible modes of transmission are through inhalation, by contact of infected saliva with an open wound or mucous membrane, and via infected corneal or solid organ transplants . Rabies virus, or related members of the genus Lyssavirus (linear single-stranded enveloped eicosahedral RNA viruses), is the causative agent that spreads from the bite site along the peripheral nerves toward the CNS. The incubation period is typically 2 to 8 weeks; however, it may be even years long, and is inversely related both to the size of the inoculum and to the proximity of the site of the bite to the CNS .
After the virus is taken up by the unmyelinated nerve ending at the bitten site, it travels toward the CNS by retrograde axoplasmic flow, at a rate of approximately 12 to 24 mm per day until the virus reaches the next neuronal cell body. The prodromal stage with migration of virus from the periphery to dorsal root ganglia causes neuropathic pain . Once inside the CNS, the viral dissemination is very rapid. The disease manifests in two distinctive forms: furious (encephalitic), which is more common, and paralytic, which is often harder to diagnose clinically, and may be confused with Guillain-Barré syndrome. Hydrophobia and spasms occur with the furious form and mental status alternates between normal periods and progressive agitation and depression, followed by deterioration of consciousness and coma. A relative sparing of consciousness with flaccid paralysis of all extremities dominates the clinical picture of paralytic rabies . The late phase of rabies cycle involves a centrifugal spread of the virus to highly innervated organs, particularly the salivary glands.
The rabies virus antigen is found mainly in the brain stem, spinal cord, and thalamus in both forms. However, it is rarely detected in the hippocampus, and an indirect functional impairment, possibly autoimmune in nature (similar to limbic encephalitis), which accompanies direct viral infection has been proposed for the encephalitic form. Peripheral nerve dysfunction, with intense inflammation of the spinal nerve roots, may be responsible for weakness in paralytic rabies. In furious rabies, progressive focal denervation is evident even in the absence of demonstrable weakness and seems to be primarily due to anterior horn cell dysfunction .
Patients with suspected rabies are investigated by collecting saliva, cerebrospinal fluid, serum, and a punch biopsy from the skin at the nape of the neck, which includes hair follicles containing peripheral nerve endings. A fluorescent antibody test to detect virus antigen in a skin biopsy and polymerase chain reaction of the saliva are very useful . The pathognomonic histologic features of rabies are the Negri bodies, although they are present in only 50% to 80% of cases proven by virus isolation methods .
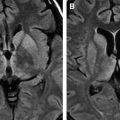
Neuroimaging studies may also be helpful, as shown by a few reports on MR imaging findings in rabies . Nonenhancing ill-defined mild hyperintensities in the brain stem, hippocampi, hypothalami, deep and subcortical white matter, as well as deep and cortical gray matter were demonstrated on T2-weighted images. Most commonly, the lesions are present in the dorsal brainstem, basal ganglia, and hippocampi ( Fig. 4 ). Enhancement along the brachial plexus of the bitten arm was noted in one patient in the early phase, suggesting inflammatory reactions in response to the presence of rabies virus during its retrograde axoplasmic flow. Mild to moderate contrast enhancement may be seen in the hypothalami and brain stem nuclei, as well as in the spinal cord gray matter, and intradural cervical nerve roots during the late phase in patients with paralytic rabies .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree