The thyroid and parathyroid glands are endocrine structures located in the visceral space of the infrahyoid neck. Imaging plays a critical role in the evaluation of patients with thyroid cancer, both in the pre and posttreatment setting. Disorders of thyroid function, that is, hyperthyroidism and hypothyroidism, are also fairly common, although imaging utilization is less frequent with these conditions. Parathyroid dysfunction results in disordered calcium metabolism. Imaging is frequently applied in the preoperative assessment of these patients undergoing parathyroidectomy; however, routine imaging in the postoperative setting is uncommon. Parathyroid carcinoma is rare; however, imaging may be used in the pre and posttreatment setting.
Key points
- •
The thyroid and parathyroid glands are endocrine structures located closely together within the neck. Pathologies affecting these glands include endocrine disorders of glandular function as well as malignancy.
- •
Thyroid cancer and potential treatment-related complications are commonly followed with imaging. Ultrasound is the imaging modality of choice for the follow-up of thyroid cancer with more advanced modalities including CT, MRI, and PET/CT reserved for more advanced disease states.
- •
Treated hyper and hypothyroidism are not commonly followed with imaging. However, these patients may be imaged for other reasons and treatment-related sequelae or complications are incidentally observed.
- •
Primary hyperparathyroidism (PHPT) is the most common parathyroid disease and also is not routinely followed with imaging. Complicated and/or recurrent cases may be evaluated with imaging and the initial posttreatment changes may be visible.
Introduction
The thyroid and parathyroid glands are endocrine structures located in the visceral space of the infrahyoid neck. Thyroid malignancy has seen a growing incidence over the last several decades without increasing mortality rate, presumably due to increasing diagnosis. Thyroid cancer is primarily treated surgically with or without adjuvant radioactive iodine (RAI) ablation. Imaging plays a critical role in the evaluation of patients with thyroid cancer, both in the pre and posttreatment setting. Disorders of thyroid function, that is, hyperthyroidism and hypothyroidism, are also fairly common, although imaging utilization is less frequent with these conditions. Parathyroid dysfunction results in disordered calcium metabolism. Imaging is frequently applied in the preoperative assessment of these patients undergoing parathyroidectomy, however, routine imaging in the postoperative setting is uncommon. Parathyroid carcinoma is rare; however, imaging may be used in the pre and posttreatment setting.
Knowledge of the expected posttreatment imaging appearances of these disease states as well as potential posttreatment complications is critical for the interpreting radiologist. This article reviews the most commonly observed imaging appearances of the thyroid and parathyroid glands after treatment for these various disease states as well as multiple possible complications.
Thyroid cancer
Epidemiology and histology subtypes
Within the United States, the incidence of thyroid cancer has been reported up to 15.7 cases per 100,000 people. Over the last several decades, this reported incidence has increased nearly 3-fold and is largely thought to be secondary to increased detection rather than a true increase in disease incidence. There is an approximately 3:1 female/male ratio and a median age of 51 at diagnosis. , Most thyroid cancers are well-differentiated tumors with the papillary subtype representing the vast majority (approximately 84%) of cases followed by the follicular subtype at 10% of cases. Papillary and follicular thyroid cancers are grouped as differentiated thyroid cancer (DTC), as the clinical management of these entities is similar.
Medullary thyroid cancer (MTC) arises from parafollicular C cells of thyroid glands and produces calcitonin. MTC is primarily sporadic, however, does occur in a hereditary pattern in around 25% of cases. , The hereditary form of MTC is often associated with multiple endocrine neoplasia (MEN) type 2, which is linked to mutations in the RET proto-oncogene. MEN type 2 has subclassification whereby Type 2A includes MTC, parathyroid adenoma or hyperplasia, and pheochromocytoma, whereas Type 2B includes MTC, pheochromocytoma, and mucosal neuroma. MTC is more aggressive than DTC and has a worse prognosis. Anaplastic thyroid cancer (ATC) is the most poorly differentiated of thyroid cancers. Although its incidence is lowest (1%–2% of all thyroid cancers), it accounts for 14% to 50% of cancer-related mortality of thyroid cancers with a median survival of 3 to 6 months.
Surgical treatment of differentiated thyroid cancer
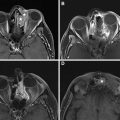
Surgery is the mainstay of treatment for differentiated thyroid cancer (DTC) with total thyroidectomy being the most common procedure ( Fig. 1 ). The goals of total thyroidectomy are to remove the entire tumor laryngeal while minimizing procedure-related complications. Near-total thyroidectomy is also performed which is the removal of all thyroid tissue except for a small amount of posterior thyroid capsule to avoid recurrent nerve injury. More recently, thyroid lobectomy has become an acceptable treatment option for patients with smaller primary tumors (<1 cm) confined to one thyroid lobe ( Figs. 2 and 3 ) and no nodules on the contralateral lobe. Lobectomy is generally a safer procedure that avoids the risk of injury to structures within the contralateral neck. In properly selected patients, there is no significant difference in recurrence rates or survival for lobectomy versus total thyroidectomy.



The primary benefits of total thyroidectomy over lobectomy are: 1) option to undergo RAI therapy for adjuvant therapy and 2) measurement of serum thyroglobulin for the surveillance of cancer recurrence. The main drawback is that patients will need to take thyroid hormone replacement medication for the rest of their life. Central or lateral compartment lymph node dissection may also be performed depending on the presence of nodal involvement (see Fig. 1 ). RAI therapy after total thyroidectomy may also be administered to ablate potential remnant thyroid tissue in intermediate or high-risk patients by American Thyroid Association (ATA) critieria.
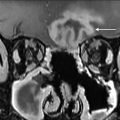
Complications of thyroid surgery include postoperative hematoma, vocal cord dysfunction, and hypoparathyroidism resulting in hypocalcemia, and typically do not result in any imaging. Vocal cord dysfunction due to postoperative recurrent laryngeal nerve injury, however, has been reported to be permanent in approximately 2.3% of patients ( Fig. 4 ). , Patients with long-standing vocal cord paralysis may go on to have surgical therapy involving the injection of any number of implants or injectable materials which may be visualized on later cross-sectional imaging ( Fig. 5 ). Hypoparathyroidism is also a serious potential complication of total thyroidectomy. Patients may present with muscle pain, tingling, or twitching of fingers or toes, irritability, depression, and dry skin. Therefore, the parathyroid glands must be actively identified during the total thyroidectomy and preserved to avoid this complication.


Posttreatment surveillance
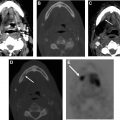
After receiving therapy, patients are followed with serial monitoring of thyroglobulin levels as well as neck ultrasound. Ultrasonography (US) has emerged as the imaging modality of choice in monitoring these patients given its relatively low cost, lack of ionizing radiation, and high accessibility as well as high accuracy in detecting nodal and thyroid bed recurrences. , The optimal sonographic technique will use a high-frequency (≥10 MHz) linear probe to achieve high resolution. The ATA recommends US 6 to 12 months after surgery to evaluate the thyroid bed as well as the central and lateral nodal compartments for recurrent disease. Subsequent follow-up intervals will vary among patients depending on individual risk factors. For patients who do receive thyroid remnant ablation, the ATA does recommend a posttherapy whole-body nuclear medicine scan to further assess staging and evaluate any remaining structural disease ( Fig. 6 ).

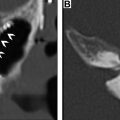
The recurrence of DTCs is fairly common and has been reported to occur in up to 30% of cases. Locoregional recurrences are often nonpalpable, making imaging follow-up extremely important. Ultrasound and possible ultrasound-guided fine-needle aspiration (FNA) biopsy have been found to be accurate in detecting recurrences within the thyroidectomy bed, even when serum thyroglobulin is undetectable. Thyroidectomy bed recurrences are most likely to appear as irregular hypoechoic nodules with internal vascularity ( Fig. 7 ). , However, many benign nodules within the postoperative bed may also appear hypoechoic, so FNA is often necessary to confirm the diagnosis. Cervical lymph node recurrences are also common and on imaging appear similar to pretreatment metastatic nodes. Typical sonographic features include abnormal rounded shape, hypoechogenicity, loss of the fatty hilum, and microcalcifications. ,

Radioiodine whole-body scintigraphy (WBS) had historically been performed in the postoperative setting and had some utility in the detection of iodine avid disease. Currently, WBS is recommended in the immediate post–RAI setting to help with disease staging and potentially detect previously unknown sites of disease. Posttherapy WBS may change the disease staging and management strategy in 8% to 9% of patients as well as to detect previously unknown sites of disease. , WBS traditionally yields planar images, which do not offer detailed anatomic information and will not be helpful in aiding preoperative planning. WBS in conjunction with fused SPECT/CT may offer improved anatomic localization as well as the detection of disease resulting in altered prognostic estimates. ,
In the case of long-term follow-up, compared to serum thyroglobulin assays and new imaging modalities such as US, WBS is fairly insensitive in the detection of DTC metastases or recurrences. , Furthermore, I-131 WBS will not detect a tumor that does not avidly take up iodine or is dedifferentiated. As a result of these limitations, WBS has largely fallen out of favor in routine postoperative surveillance for patients with thyroid cancer. The ATA does not recommend routine follow-up WBS in patients who had a posttherapy scan showing no uptake outside the thyroidectomy bed.
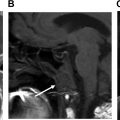
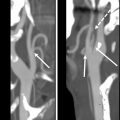
Computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography/computed tomography (PET/CT) are also not routinely used in posttreatment surveillance of DTC, however, may play a role in cases of distant metastatic disease, recurrences that are invading adjacent structures, or other situations such as widespread nodal disease in which US may be inadequate for full disease mapping. Patients presenting with concerning symptoms such as hoarseness, dysphagia, or a rapidly growing neck mass also warrant further evaluation with CT or MRI to assess for locoregional invasion ( Fig. 8 ).