Benign uterine diseases are common gynecologic conditions affecting women of all ages. Ultrasonography is traditionally the first-line imaging technique but patients are increasingly referred to magnetic resonance (MR) imaging because it is more accurate for diagnosis and patient management. This article highlights the added value of MR imaging in the diagnosis of the most common benign uterine diseases, describes therapeutic options, and delineates the role of MR imaging in treatment planning.
Key points
- •
Benign uterine disease mainly comprises adenomyosis and leiomyomas, common gynecologic conditions affecting women of all ages.
- •
Magnetic resonance (MR) imaging is the most accurate imaging modality for detection and localization of benign uterine disease and its mimics.
- •
MR imaging is the diagnostic tool of choice for pretreatment evaluation, assessing potential procedural risk and predicting treatment response, and monitoring treatment outcomes.
Introduction
Benign uterine diseases, such as adenomyosis and leiomyomas, are common gynecologic conditions affecting women of all ages. Although ultrasonography (US) is the first-line imaging technique in the examination of the uterus, magnetic resonance (MR) imaging has become very useful and is the most accurate tool in lesion diagnosis and patient management. Benign uterine disease may manifest with atypical features and can mimic malignancy, making the correct diagnosis a challenge. , , MR imaging also assists in the triage of symptomatic patients to the most appropriate treatment modality, including surgery (hysterectomy or myomectomy), interventional procedures (uterine artery embolization), and medical therapy. Hysterectomy is curative, but uterus-sparing therapies are a valid alternative for eligible women.
This article highlights the utility of MR imaging in the diagnosis of the most common benign uterine diseases, such as leiomyomas and adenomyosis, discusses their typical and atypical MR imaging findings, describes the therapeutic options, and delineates the role of MR imaging in treatment planning.
Magnetic resonance imaging protocol
Clinical guidelines for diagnosis and management of patients with leiomyomas are extensive. , , Recently, recommendations for MR imaging were proposed by the European Society of Urogenital Radiology (ESUR).
Patient Preparation
Fasting and the use of an antiperistaltic agent such as Hyoscine Butylpromide or glucagon is useful to minimize motion artifacts related to small bowel peristalsis. A moderately full bladder is recommended to reduce artifacts related to bladder filling.
Imaging Protocol
High-resolution thin-section images acquired at 1.5 T or 3.0 T are recommended. The optimal protocol, according to recent ESUR guidelines, is summarized in Table 1 .
| T2WI | High-resolution T2 sequences in the axial, oblique, sagittal, and coronal planes. The axial oblique T2W sequence perpendicular to the corpus of the uterus is particularly useful to evaluate the location of the lesion relative to the endometrial cavity |
| T1WI | Axial T1W sequence of the pelvis with and without fat suppression. Axial T1W sequence is useful to evaluate the presence of fat or blood contents and can be used to detect the presence of lymph nodes and bone marrow abnormalities |
| Large-FOV T1WI/T2WI | Large-FOV T1W or T2W sequence of the upper abdomen. It allows visualization of secondary signs of pelvic mass effect, such as hydronephrosis, and malignant disease, such as lymph nodes or peritoneal carcinomatosis |
| Contrast-enhanced T1WI | Contrast-enhanced axial T1WI of the pelvis with fat saturation. It allows further lesion characterization, vascularization, and its differentiation from an adnexal mass |
| Dynamic contrast injection/MR angiography | Dynamic contrast injection/MR angiography is recommended if uterine artery embolization may be a possibility in order to evaluate uterine artery anatomy and collateral gonadal arterial supply |
| DWI | DWI is not mandatory but has shown added value for lesion characterization and distinction between leiomyoma and leiomyosarcoma |
Leiomyomas
Epidemiology, Pathophysiology
Leiomyomas are the most common benign uterine tumors, affecting up to 20% to 30% of reproductive-aged women. They are benign neoplasms of unknown cause composed of multiple layers of smooth muscle fascicles and fibrous connective tissue anchored in the muscular wall of the uterus. These tumors may be solitary or, most frequently, multiple. The size of leiomyomas is variable and influenced by estrogen and progesterone; they often grow during pregnancy and with oral contraceptive use and regress after menopause. They may be asymptomatic, but 20% to 50% of women with leiomyomas present with symptoms such as menorrhagia, dysmenorrhea, pressure, urinary frequency, pelvic and back pain, and dyspareunia.
Location
Leiomyomas generally involve the myometrium of the uterine corpus and are classified by their location as submucosal, intramural, and subserosal. In accordance with the International Federation of Gynecology and Obstetrics (FIGO) classification system, they can be further subdivided into 8 categories ( Fig. 1 ). Pedunculated lesions can become detached from the uterus, receiving blood supply from other adjacent structures, and are called parasitic leiomyomas. , Leiomyomas not related to the myometrium may be located in the cervix and in the round or broad ligaments.

Leiomyomas may occur in unusual locations, such as diffuse peritoneal leiomyomatosis, intravenous leiomyomatosis, or benign metastasizing leiomyoma. Peritoneal leiomyomatosis is characterized by multiple lesions along the peritoneal surfaces, probably caused by the iatrogenic dissemination throughout the peritoneal cavity after surgery. A previous history of hysterectomy for leiomyomas or a diagnosis of uterine leiomyomas may point to the correct diagnosis. Intravenous leiomyomatosis has an aggressive intravascular growth pattern within intrauterine and systemic veins.
Imaging Features
Ultrasonography
US is usually the initial imaging study.
On US, a typical leiomyoma is a well-defined mass, often shadowing at the margin and/or producing internal linear shadowing. The echogenicity varies from hypoechoic to hyperechoic in relation to the myometrium. Dystrophic calcifications can be seen particularly in postmenopausal women. On color Doppler US, circumferential blood flow around the lesion is often seen.
Magnetic resonance imaging
US can be limited by coexisting pelvic diseases, uterine anomalies, unusually small or large tumors, and tumor location. Thus, MR imaging is considered the most accurate imaging modality to detect, locate, and characterize myometrial lesions before patient management. , MR imaging has reported sensitivities and specificities of 94.1% and 68.7%, respectively, for uterine leiomyomas. , ,
On MR imaging, most leiomyomas, without any degeneration, are easily recognized as well-delineated round or ovoid lesions homogeneously hypointense on T2-weighted imaging (T2WI) related to the outer myometrium and isointense on T1-weighted imaging (T1WI), with heterogeneous and variable enhancement. The presence of a T2-hyperintense rim indicates a pseudocapsule of edema caused by dilated lymphatic vessels and veins ( Fig. 2 ). Typical leiomyomas do not have restricted diffusion, showing a low signal intensity both on diffusion-weighted imaging (DWI) and on the corresponding apparent diffusion coefficient (ADC) map, known as the blackout phenomenon (see Fig. 2 ).

There are 2 subtypes of leiomyoma.
Cellular leiomyoma is a histologic subtype characterized by more compact smooth muscle cells with little or no collagen; on MR imaging it shows higher signal intensity on T2WI and avid enhancement ( Fig. 3 ). The intermediate T2 signal and avid enhancement of cellular leiomyomas can make it difficult to differentiate them from leiomyosarcoma (see Fig. 3 ). In a retrospective study of 51 patients with a single myometrial lesion at MR imaging, Thomassin-Naggara and colleagues suggested that the evaluation of DWI and ADC (value cutoff 1.23 × 10 −3 mm 2 /s) may limit the misdiagnosis of uterine sarcoma as leiomyoma with diagnostic accuracy of 92.4% (see Fig. 3 ).

Lipoleiomyoma occurs in 0.03% to 0.2% of women, generally in the postmenopausal population. Lipoleiomyomas are composed of smooth muscle, adipose tissue, and fibrous tissue. The signal intensity of the fat components is typical high on T1WI and T2WI with loss of signal intensity on fat-saturated sequences.
When leiomyomas enlarge and outgrow their blood supply (≥5 cm), they may degenerate. , The types of degeneration are hyaline, cystic, myxoid, hemorrhagic, and calcific ( Table 2 ).
| T2WI Signal | T2WI Border | T1WI | C+ | DWI | |
|---|---|---|---|---|---|
| Type of Leiomyoma | |||||
| Nondegenerated | Hypointense | Well defined | Isointense | Variable | Hypointense |
| Cellular subtype | Hyperintense | Well defined | Isointense | Vivid enhancement | Hyperintense |
| Lipoleiomyoma subtype | Variable: fat component (hyperintense) | Well defined | Variable: fat component (hyperintense) | Variable | Variable due to fat content |
| Degeneration Type | |||||
| Hyaline | Hypointense | Well defined | Isointense | Moderate enhancement | Hypointense |
| Cystic | Hyperintense | Well defined | Hypointense | No enhancement | Hypointense |
| Myxoid | Very hyperintense | Well defined | Hypointense | Delayed enhancement | Hypointense |
| Hemorrhagic | Variable | Well defined | Hyperintense | Variable | Variable due to blood content |
| Calcific | T2 hypointense | Well defined | Hypointense | No enhancement | Hypointense |
| Leiomyosarcoma | T2 dark areas | Nodular border | Presence of High T1 SI related to blood | Enhancement with central necrosis | High DWI/low ADC |
Hyaline degeneration is the most common type (60%). Leiomyomas with hyaline degeneration have low signal intensity on T2WI, an appearance similar to that of nondegenerated leiomyomas; however, they enhance to a lesser degree than standard leiomyomas ( Fig. 4 A, B).

Cystic degeneration (4%) is characterized by the presence of cystic areas with high signal intensity on T2WI that do not enhance ( Fig. 4 C, D).
Myxoid degeneration is rare and depends on the presence of gelatinous intralesional foci at gross examination that contain hyaluronic acid–rich mucopolysaccharides. Leiomyomas with myxoid degeneration have an extremely high signal intensity on T2WI and enhance well except for foci of mucinous lakes or clefts. Delayed and prolonged contrast enhancement is caused by the presence of myxoid stroma ( Fig. 4 E, F).
Hemorrhagic degeneration (red degeneration) is caused by hemorrhagic infarction resulting in coagulative necrosis and is associated with pregnancy and oral contraceptives. Red degeneration may also occur after uterine artery embolization (UAE). On MR imaging, the signal intensity is variable: peripheral or diffuse hyperintensity on T1WI and inhomogeneous signal intensity on T2WI with or without the hypointense rim. The T1 hyperintensity is caused by the proteinaceous content of the blood and/or by the T1-shortening effect of methemoglobin. When the hyperintensity is peripheral, it may be caused by thrombosis of the vessels that surround the lesion ( Fig. 4 G, H).
Calcific degeneration is associated with end-stage hyaline degeneration and post-UAE treatment changes. On MR imaging the calcific components produce signal voids on all sequences.
Smooth muscle tumor of uncertain malignant potential (STUMP) is a rare heterogeneous tumor that cannot be definitively classified histologically as leiomyoma or leiomyosarcoma. Multiple subtypes have been identified according to nuclear atypia, mitotic rate, and necrosis. On MR imaging, there are no specific features, because STUMP mimics both typical leiomyoma and leiomyosarcoma. After removal they have a high rate of recurrence (7.3%–12.5%) and may recur as low-grade leiomyosarcoma; therefore, long-term follow-up is needed. ,
Pitfalls to avoid on magnetic resonance imaging
- •
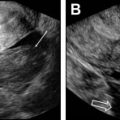
Distinction between an ovarian mass and a uterine mass: it might be hard to distinguish uterine leiomyoma from a fibrous ovarian mass. In a ovarian mass, sharp angles between the ovary and the lesion are known as the beak sign. In contrast, in a uterine mass, a normal ovary is seen separate from the mass. The claw sign (uterine tissue draped around the mass) (see Fig. 4 C) or the bridging-vessels sign (enlarged and tortuous vessels extending from the uterus to the lesion) suggest the diagnosis of a uterine mass (see Fig. 4 H; Fig. 5 ).

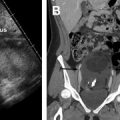
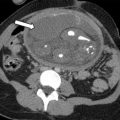
Fig. 5
Indeterminate origin of a mass: ovarian versus uterine. Axial T2-weighted image shows a well-defined left parauterine mass with low signal intensity that could resemble either a uterine leiomyoma or ovarian fibroma. In this case, the left ovary ( black arrow ) is normal and separate from the mass. Enlarged and tortuous vessels extended from the uterus to supply the mass ( white arrows , bridging vessels sign), suggesting the diagnosis of uterine leiomyoma.
- •
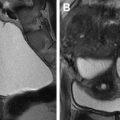
Focal myometrial contractions: contractions may appear as low-T2-signal myometrial masses and may simulate uterine leiomyomas. Because they are transient and usually do not persist during the entire examination they can be easily differentiated from leiomyomas ( Fig. 6 ).

Fig. 6
Transient myometrial contraction. ( A ) Sagittal and ( B ) axial T2-weighted fat-saturated images. Axial T2-weighted image shows hypointense bands perpendicular to the junctional zone (JZ) in the anterior myometrium ( arrows , B ). This finding was absent on previous sagittal T2-weighted image that shows normal uterus with thin and distinct JZ ( arrow , A ), confirming the diagnosis of transient myometrial contraction.
- •
Endometrial polyp: submucosal leiomyoma may be mistaken for an endometrial polyp. Polyps have heterogeneous or high signal on T2WI, in contrast with pedunculated submucosal leiomyomas, which are low signal on T2WI and have a stalk that arises from the myometrium.
- •
Adenomyomas: discussed later.
- •
Leiomyosarcomas: it is critical to differentiate a benign leiomyoma from leiomyosarcoma. Although leiomyosarcomas arise de novo and have no biological link to leiomyomas, they may present with symptoms and imaging features similar to leiomyomas. Large size and rapid growth are unreliable signs of malignancy. Growth of a uterine mass after menopause and increased lactate dehydrogenase (LDH [level]), particularly LDH isozyme type 3, is suspicious for leiomyosarcoma. Endometrial sampling may aid diagnosis of uterine sarcoma but sensitivity is limited because of the myometrial origin of the tumor. , Although no single MR imaging feature can reliably distinguish leiomyosarcomas from atypical leiomyomas, a combination of MR imaging features may improve the diagnostic performance of MR imaging for the correct diagnosis of leiomyosarcoma. In a study from Lakhman and colleagues, the combination of 3 or more of 4 discriminative features, including nodular borders, hemorrhage (high signal intensity on T1WI), T2-weighted (T2W) dark areas, and central areas of nonenhancement, was associated with an improved sensitivity and specificity for the diagnosis of leiomyosarcoma ( Fig. 7 ). Uterine sarcoma also shows rapid early enhancement of the solid components and restricted diffusion. Diffusion restriction alone is insufficient for diagnosis because there is considerable overlap in ADC values between leiomyomas and leiomyosarcoma; leiomyomas may show restricted diffusion, especially cellular leiomyomas (see Fig. 3 D, E). Notably, the presence of restricted diffusion and a T2 blackout effect is highly specific for a leiomyoma (see Fig. 2 ). More recently, Thomassin-Naggara and colleagues reported that, using a recursive model combining T2 signal intensity, b1000 images, and ADC map with a cutoff value 1.23, MR imaging achieved 92.4% accuracy in distinguishing benign and uncertain or malignant myometrial tumors. The investigators concluded that DWI may be of interest to distinguish between uterine sarcomas and benign leiomyomas. Fig. 8 shows an algorithm to help differentiate leiomyoma and leiomyosarcoma.