In this article, the authors review the optimal imaging protocols for ultrasound and MR imaging of suspected endometriosis, review the compartmental approach to dictating these examinations, discuss the diagnostic criteria for endometriosis detection by anatomic site and the differential diagnosis, review pearls and pitfalls in diagnosis, and review what the referring physician needs to know.
Key points
- •
Endometriosis is a common disease.
- •
In women of reproductive age with pelvic pain, dysmenorrhea, dyspareunia, or infertility, endometriosis as a cause should be considered.
- •
Detection of endometriosis on imaging can be subtle; therefore, a targeted approach to imaging evaluation is recommended.
- •
Targeted ultrasound and MR imaging techniques are effective in the detection of endometriosis.
Introduction
Endometriosis is a common, benign disorder defined as the presence of endometrial tissue at extrauterine sites, typically involving the pelvis. The cause and pathogenesis of endometriosis are incompletely understood and appear to be due to multifactorial causes, including the presence of ectopic endometrial tissue, altered immunity, disrupted cell proliferation and apoptosis, and disordered endocrine signaling and genetics. The prevalence estimates of endometriosis vary, with 1% of all women who undergo major gynecologic surgery having evidence of endometriosis, and 9% to 50% of all women undergoing laparoscopy for infertility having evidence of endometriosis. , It is estimated that there is a 10% prevalence of endometriosis in women of reproductive age. The classic symptoms of endometriosis are dysmenorrhea, pelvic pain, dyspareunia, and/or infertility.
Typically, once endometriosis is clinically suspected, patients are evaluated on physical examination for tenderness in commonly involved pelvic regions (cul-de-sac, rectovaginal space, uterine body, and uterosacral ligaments). Laboratory tests are limited in the diagnosis of endometriosis (CA-125 has a detection rate of only 54% in patients with severe endometriosis and is neither sensitive nor specific for the diagnosis). The gold standard for diagnosis of endometriosis has traditionally been surgical visualization and biopsy; however, in recent years, diagnostic laparoscopy (surgery performed in order to diagnose endometriosis, not to treat endometriosis) is becoming less common at university hospitals. Replacement of diagnostic laparoscopy by noninvasive imaging is due to the improvement in diagnostic capability of preoperative imaging (typically ultrasound [US] and MR imaging) for the detection of endometriosis.
Endometriosis typically manifests in 3 ways: ovarian endometriomas, superficial peritoneal implants, and deep infiltrating endometriosis (DIE). DIE is defined as a solid endometriotic implant more than 5 mm deep to the peritoneum. Both ovarian endometriomas and DIE can be detected accurately with imaging. Accurate imaging is important for confirming the presence of disease and for guiding treatment decisions.
Treatment of endometriosis is complicated and includes conservative approaches combined with medical therapies or surgical intervention. The treatment decisions are individualized and consider the patient’s clinical presentation, duration and type of symptoms, the disease extent, and location as determined by imaging, the patient’s age, reproductive wishes, medication cost/side effects, and surgical cost/patient candidacy for surgery. The American Society for Reproductive Medicine Practice Committee states that “endometriosis should be viewed as a chronic disease that requires a lifelong management plan with the goal of maximizing the use of medical treatment and avoiding repeated surgical procedures.” To that end, many patients are stratified for medical treatment with or without surgical treatment based on the severity of the symptoms or imaging findings and desire for child-bearing, with first-line medical therapy typically including nonsteroidal anti-inflammatory drugs and hormonal (contraceptive) agents, with more severe symptoms being treated with gonadotropin-releasing hormones and/or surgery (again, based on clinical symptoms, disease severity, and patient factors). The resection of endometriomas and endometriotic implants at the time of surgery is important for symptom control and preservation of fertility. Thorough preoperative planning is essential for complete endometriosis excision.
In this article, the authors review the optimal imaging protocols for US and MR imaging of suspected endometriosis, review the compartmental approach to dictating these examinations (with a focus on mapping of disease before surgical intervention), discuss the diagnostic criteria (sensitivity and specificity of US and MR) for endometriosis detection by anatomic site, discuss the differential diagnosis, and review pearls and pitfalls in diagnosis and what the referring physician needs to know.
Normal anatomy and imaging technique
Endometriosis most commonly occurs in the following locations in the pelvis: the ovaries, uterus, fallopian tubes, uterosacral ligaments, broad ligaments, round ligaments, cul-de-sac, bladder, ureters, rectovaginal septum, and rectosigmoid colon. The frequency of endometriotic implants in each of these locations, with the corresponding sensitivity and specificity of detection in these regions by specialized endometriosis US and MR, is listed in Table 1 .
| Location | Frequency Present in This Location at Laparoscopic Evaluation, % | Specialized Endometriosis US Detection , , | MR Imaging Detection , , |
|---|---|---|---|
| Retrocervical region/uterosacral ligaments | 60–85 | 53%–64% sensitivity 93%–97% specificity | 86% sensitivity 84% specificity |
| Uterus | 40 | n/a | 86% sensitivity 84% specificity |
| Ovaries | 20–40 | 83% sensitive 89% specific | 90% sensitive 98% specific |
| Bladder | 3–20 | 55% sensitivity 93.5% specificity | 75%–87% sensitive 99%–100% specific |
| Rectosigmoid colon | 9.9–37 | 90% sensitive 96% specific | 85%–91% sensitive 72%–89% specific |
| Rectovaginal septum | 11 | 81% sensitive 95% specific | 81% sensitive 86% specific |
| Vagina | 14.5–30 | 57% sensitive 99% specific | 77%–79% sensitive 76%–93% specific |
| Round ligaments | 0.3–14 | n/a | 20%–40% sensitive 30% specific |
| Ureters | 0.01–1 | Limited data: 92% sensitivity 100% specificity | 83% sensitive 98.6% specific |
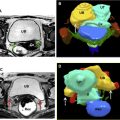
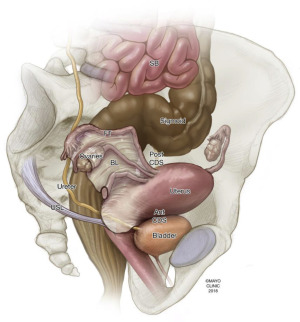
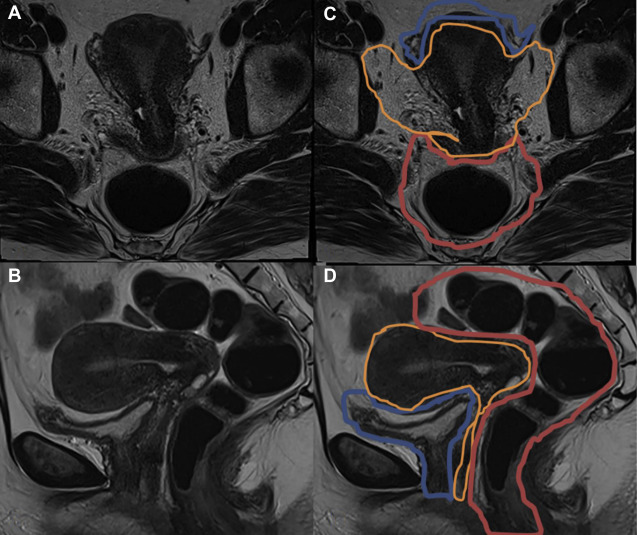
These anatomic regions can be subdivided according to functional and clinical relevance into anterior, middle, and posterior compartments. The anterior compartment includes the insertion site of the ureters, the bladder, the vesicouterine pouch, and the vesicovaginal pouch. The middle compartment contains the uterine body, fallopian tube, and uterine ligaments. The posterior compartment contains the uterosacral ligaments, rectovaginal septum, anterior rectal wall, and sigmoid colon. A cartoon depiction of the regions typically involved by deep endometriosis is shown in Fig. 1 . The corresponding normal anatomy for the anterior compartment, middle compartment, and posterior compartments is shown for US ( Fig. 2 ) and MR imaging ( Fig. 3 ).
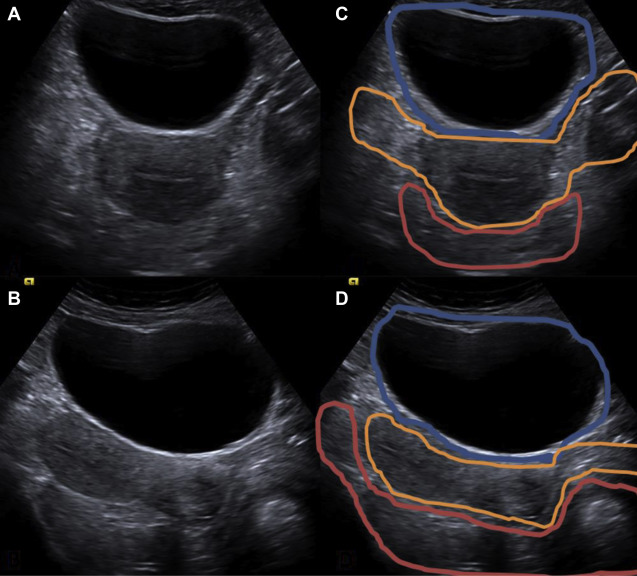
Ultrasound
Transvaginal ultrasound (TVUS) is typically the initial imaging evaluation performed in patients with pelvic pain and infertility or when there is clinical suspicion for endometriosis. This examination is very accessible compared with MR imaging, and the sensitivity and specificity for detection of ovarian endometriomas and lesions in the rectal wall are high. However, there is controversy in the lack of reproducibility in the community of the US technique described by specialized tertiary care centers, and the inability of nontertiary care centers to reproduce the high sensitivity and specificity rates reported in the literature.
The best means of detection of deep endometriosis on US require a more involved US protocol than that which is traditionally used for screening TVUS. This “specialized endometriosis” US protocol involves techniques described by the American Institute of Ultrasound in Medicine (AIUM) and the International Deep Endometriosis Analysis (IDEA) groups, including use of a targeted physical examination before obtaining the images, the routine TVUS protocol combined with a dedicated targeted compartmental transvaginal sonogram (described in Box 1 ), with attention primarily to the posterior compartment (uterosacral ligaments, rectovaginal septum, and rectum), with use of the “sliding organ” maneuver to detect subtle adhesions (see Box 1 ), with additional more specialized techniques also considered (tenderness-guided transvaginal sonography; rectal-water transvaginal sonography). For detection of uterovesicular adhesions/implants, the sliding maneuver is as follows: the transvaginal probe is placed in the anterior fornix and the uterus is moved between the probe and 1 hand of the operator that is placed over the suprapubic region. If the posterior bladder slides freely over the anterior uterine wall, then the sliding sign is positive and the uterovesical region is classified as nonobliterated. If the bladder does not slide freely over the anterior uterine wall, then the sliding sign is negative and the uterovesical region is classified as obliterated.
- 1.
Before the ultrasound, a targeted physical examination should be performed by the physician (radiologist or gynecologist) reporting fixation of uterus, cervical/vaginal tenderness, visible endometriosis implants.
- 2.
Standard ultrasound Transabdominal/Transvaginal pelvic examination (as described by the Practice Parameter of the AIUM). This includes measuring in 2 tangential planes and documenting the following: (1) the uterus (including uterine size, shape, and orientation; the endometrium; the myometrium; and the cervix); (2) the adnexa (ovaries and fallopian tubes); and (3) the cul-de-sac (including attempts to evaluate bowel posterior to the uterus).
- a.
This is considered “Step 1” of the ultrasound evaluation, looking for signs of adenomyosis or ovarian endometriomas.
- a.
- 3.
Targeted compartmental sonographic evaluation as described by the IDEA group, evaluating the anterior, middle, and posterior compartments. For this component of the evaluation (steps 2–4 of the evaluation), the following are required:
- a.
Step 2 is the evaluation of transvaginal “soft markers” (ie, site-specific tenderness and ovarian mobility).
- b.
Step 3 is the evaluation of the pouch of Douglas using the ultrasound “sliding sign.”
- c.
Step 4 is the assessment for deep infiltrating endometriosis nodules in the anterior and posterior compartments.
- a.
- 4.
Optional: Tenderness guided transvaginal sonography:
- A.
Evaluation of the cul-de-sac, bowel wall, and rectovaginal septum while gently palpating with the probe to elicit the areas of tenderness.
- B.
This technique involves incremental evaluation of the pelvis, beginning with the ovary, moving posteriorly toward the cul-de-sac slowly, while palpating and evaluating regions of discomfort. In the medial region, the uterosacral ligaments are assessed, and the posterior cervical lip and rectovaginal septum are evaluated, along with the rectum, and the posterior cervix. The cervix should be moved slightly with the probe, while observing the movement of the cervix and uterus with the operator’s hand. Fixated movement will suggest adhesions. The bowel should be observed for peristalsis (any regions without peristalsis will suggest implants in those regions).
- A.
In specialized tertiary care centers where attention to this meticulous technique is followed, US has the potential for high sensitivity and specificity (as high as 95% sensitivity and 96% specificity) , , , , in detection of posterior compartment and bowel endometriosis (see Table 1 ). Other techniques that have been suggested for improved sensitivity include using a transrectal approach and/or a bowel preparation for better visualization of the bowel wall as well as instillation of extra gel in the vagina. Limitations of sonographic technique, despite meticulous efforts, include anterior compartment detection of endometriosis (bladder and vesicouterine pouch detection) and detection within the middle compartment (torus uterinus and round ligaments). However, because posterior compartment endometriosis is the most frequently involved compartmental region by deep endometriosis, this modality, when performed with specialized endometriosis sonographic technique, represents an effective screening examination in this population.
MR Imaging
MR imaging of the pelvis is frequently performed for the detection of endometriosis, either as the second-line imaging examination (after US) for the detection/confirmation of endometriosis or as the initial examination in a patient for whom there is a high clinical suspicion for endometriosis. MR imaging can be performed with either a 1.5-T or 3-T magnet, using a high-resolution phased-array surface coil for improved resolution. There is no consensus regarding whether to perform the examination around the timing of the patient’s menstrual cycle. Fasting before the examination for 4 hours is typically recommended in order to empty the upper gastrointestinal tract so that the patient will not have material to vomit with supine positioning, with antiperistaltic administration, or after intravenous (IV) contrast administration. The bladder should be moderately full to improve detection of implants in the anterior compartment. Some practices recommend a bowel enema to clear out the colon; however, others have reported equal sensitivity and specificity without an enema. ,
Patients are positioned supine on the scanner, with abdominal strapping after phased coil array placement. Antiperistaltic agents are generally recommended; however, the type of agent (oral agents, nonoral agents), dose, and route (intramuscular, subcutaneous, or IV) is debated; at New York University, the authors give 1 mg IV glucagon immediately before the acquisition of the 3-plane T2-weighted images (because these are the most sensitive sequences for the detection of subtle endometriosis, after which the glucagon often wears off). The administration of vaginal (gel) and rectal contrast (gel or water) is optional; the authors have the patient self-administer vaginal gel via a syringe on the table before placing the phased array coil.
For MR imaging sequences ( Table 2 ), 3-plane 2-dimensional T2 turbo spin echo (TSE) -weighted images are standard for acquisition; 3-dimensional T2-weighted images are considered optional (because of their decreased contrast resolution). Half-Fourier acquisition in coronal plane single-shot TSE images is considered standard in addition to true T2 TSE-weighted images, particularly because these allow for a larger field of view to assess for ureteral endometriosis and hydronephrosis in the kidneys. T1-weighted images without and with fat suppression are standard, because the fat suppression is useful for the detection of subtle foci of hemorrhage, which may be obscured on non–fat-saturated images. Dixon technique or conventional in- and out-of-phase T1-weighted images are useful for the differentiation of fat-containing lesions, such as dermoid cysts from endometriomas, both of which have high signal on non–fat-saturated T1-weighted images. The addition of diffusion-weighted images, susceptibility-weighted images, and postcontrast images is considered optional in the imaging for endometriosis.
| Sequence | Plane | Slice Thickness, mm | Field of View, cm | Matrix | Repetition Time (ms) | Echo Time (ms) | No. Averages | B Value (s/mm 2 ) | Contrast Delay | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient self-administers 3 × 12-cc syringes; vaginal contrast (US gel) | ||||||||||
| Tru FISP Scout | T1/T2 | 3 plane Cor/Sag/Ax | 7-8 | 40 | 192 × 144 | 700 | 1.13 | 1 | ||
| Inject IV glucagon, do T2 sequences immediately after injection | ||||||||||
| T2 TSE | T2 | Sag | 4 | 30–35 (adjust to body habitus) | 512 × 256 | 4870 | 102 | 2 | ||
| HASTE | T2 | Cor | 5 | 320 × 256 | Infinite | 93 | 1 | |||
| T2 TSE | T2 | Ax | 4 | 448 × 291 | 5110 | 99 | 2 | |||
| Diffusion | T2 | Ax | 6 | 160 × 120 | 6600 | 70 | 2 | b0, b50, b100, b400, b800 | ||
| In/out | T1 | Ax | 5 | 256 × 232 | 168 | 1.1/2.2 (3T) | 1 | |||
| Pre-VIBE | T1 | Sag | 2.5 | 256 × 205 | 3 | 1.22 | 1 | |||
| Pre-VIBE | T1 | Ax | 3 | 256 × 179 | 2.6 | 1.05 | 1 | |||
| Post-VIBE | T1 | Sag/Ax | 2.5 | 256 × 205 | 3 | 1.22 | 1 | 30, 60, 180 s | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree