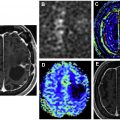
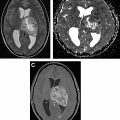
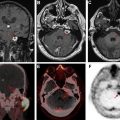
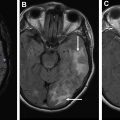
It is essential to be aware of widely accepted criteria for grading of treatment response in both high-grade and low-grade gliomas. These criteria primarily take into account responses of measurable and nonmeasurable lesions on T2-weighted, fluid-attenuated inversion recovery, and postcontrast images to determine a final category of response for the patient. The additional role that other advanced imaging techniques, such as diffusion and perfusion imaging, can play in the surveillance of these tumors is discussed in this article.
Key points
- •
The 2016 revised World Health Organization classification and grading system of gliomas includes additional information obtained from molecular biomarkers.
- •
Modified Response Assessment in Neuro-oncology (RANO) criteria divide treatment response in gliomas into complete response, partial response, progressive disease, and stable disease based on assessment of measurable and nonmeasurable lesions.
- •
Pseudoprogression is identified radiographically when tumors undergo growth similar to true disease progression defined by RANO criteria but occurring between 3 and 6 months’ after treatment, typically with concurrent radiation treatment and temozolomide.
- •
Pseudoprogression has been shown to involve reduced relative cerebral blood volume (rCBV) on gadolinium-based dynamic susceptibility contrast perfusion magnetic resonance imaging scans in contrast with increase in the rCBV index in true progression.
Introduction
Gliomas are the most ubiquitous neoplasms of the central nervous system. According to the Central Brain Tumor Registry of the United States (CBTRUS), the reported average age-adjusted incidence rate for all benign and malignant central nervous system (CNS) tumors is 23.41, with glioblastomas being the most common malignant tumor. Glioblastomas account for 14.6% of all tumors of the CNS. Before 2016, prognosis was based on the interpretation of hematoxylin-eosin–stained sections observed on light microscopy. The revised World Health Organization (WHO) classification and grading system of 2016 was based on the added information obtained from molecular biomarkers. , The major molecular markers that differentiate the histologic types of gliomas and their prognostic significance are summarized in Table 1 .
| Molecular Marker | Implication for Follow-up Imaging and Treatment Strategies |
|---|---|
| IDH1 and IDH2 mutations | Marker for astrocytic tumors. IDH wild-type (without mutations) is associated with an aggressive course. IDH mutant tumors include diffuse and anaplastic astrocytic gliomas and oligodendroglial tumors |
| 1p-19q codeletion | Confirming marker for oligodendroglial tumors if associated with IDH mutations |
| Alpha-thalassemia/mental retardation X-linked syndrome expression | Associated with IDH wild-type astrocytic tumors. Intermediate prognosis |
| TERT promotor mutations | IDH wild-type with TERT mutations is associated with poor prognosis |
| EGFR amplification | EGFR amplification and overexpression is seen in 40% of GBMs. EGFR-targeted therapies have led to a specific imaging techniques using PET-CT radiopharmaceuticals and experimental MR imaging contrast agents |
| MGMT promotor methylation | In the diagnostic schema for GBM, MGMT promoter methylation and IDH mutant GBMs denote better prognosis |
| Chromosome 7 gain and chromosome 10q loss | As in the TERTp mutations, the presence of this mutation is associated with poor prognosis in tumors that behave like GBMs |
| H3 K27M histone mutation | Identifier of diffuse midline gliomas, which are aggressive grade 4 tumors with no targeted therapies and universally poor outcomes |
Grading of gliomas combines the observed prognosis from histologic data and expected course of the tumors. Grade I tumors can be excised with low potential for recurrence. Grade II tumors show low levels of proliferative activity but are infiltrative with a potential to dedifferentiate to higher grades of the tumor. Grade III lesions show cellular atypia with higher proliferative indices and are frequently associated with recurrence. Grade IV tumors are associated with active mitoses and a propensity for necrosis. Grade IV tumors are rapidly evolving tumors with a relentless progressive course and invariably have a fatal outcome.
Treatment and response assessment of high-grade gliomas
The International Society of Neuropathology established the Haarlem guidelines to consider how the molecular profile of a tumor can be incorporated into the current grading of tumors. An understanding of this schematic diagnosis is essential because it reflects on the anticipated treatment protocols and hence the prognosis of the tumors.
Preoperative functional imaging including tractography, precision surgical navigation techniques aided by magnetic resonance (MR) imaging studies, intraoperative MR imaging, ultrasonography, and 5-aminolevulinic acid are the investigative tools available to neurosurgeons to aid in maximizing tumor excision. MR imaging remains the mainstay in the assessment of residual tumor in the immediate postsurgical period and early in the follow-up following surgery. Studies with and without contrast are recommended together with diffusion scans.
Treatment Strategies
Surgical management is considered after radiographic assessment and determining the functional risks to the patient. The Karnofsky Performance Scale is often used to help make this determination. Although surgical intervention is needed to establish a diagnosis with biopsy, gross total resection reduces the bulk of tumor and is the goal before initiating pharmacotherapy. The age of the patient and the performance scores dictate the clinical outcomes.
Radiotherapy and chemotherapy are the next line of defense to mitigate the progression of malignant gliomas. The age of the patient, extent of tumor resection, and the performance score play key roles in choosing the treatment paradigm before commencement of radiotherapy. MR imaging examinations performed after surgical resection are used to determine the extent of residual tumor (ie, gross tumor volume, based on the enhancing portions that remain in the surgical bed). T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences play key roles in the demarcation of the irradiated margin. Inclusion of this zone surrounding the tumor bed is defined as the clinical target volume. , Functional correlation using F 18 -fluorodeoxyglucose (FDG)-PET is increasingly being advocated to accurately delineate metabolic activity beyond the enhancing margin of the tumor or included in the radiation field. ,
Alkylating agents and nitrosourea compounds are the mainstay of cytotoxic chemotherapy for malignant gliomas. The oral alkylating agent temozolomide is the frontline chemotherapeutic agent for the treatment of astrocytic tumors such as diffuse astrocytomas, glioblastomas, and diffuse midline gliomas because of its favorable penetration through the blood-brain barrier, good safety profile, and documented favorable effect on the median progression-free survival (PFS) and median overall survival.
Nitroureas are the second line of chemotherapeutic agents and include lomustine, carmustine, nimustine, and fotemustine. Of these agents, lomustine (together with procarbazine and vincristine) and carmustine (used as a wafer placed locally on the margins of the surgical cavity) have been used successfully with WHO grade III and IV gliomas and recurrent gliomas. ,
Antiangiogenic agents inhibit vascular endothelial growth factors (VEGFs) and play an important role in the treatment of recurrent glioblastoma. The titular representative of this class of drugs, bevacizumab (Avastin, a synthetic monoclonal antibody that inhibits tumor neoangiogenesis by targeting VEGF), often results in misleading decrease in enhancement of the treated tumors, termed tumor pseudoresponse.
Immunotherapy is an emerging frontier in the management of gliomas. Several agents have been included in clinical trials and research is still ongoing. Immune checkpoint inhibitors primarily target immunosuppressive factors such as programmed cell death 1 ligand (PD-L1), cytotoxic T-lymphatic antigen-4 (CTLA-4), and indolamine 2,3-dioxygenase (IDO). Nivolumab and pembrolizumab are PD-L1 pathway inhibitors. Ipilimumab is an anti-CTLA-4 antibody currently approved by the US Food and Drug Administration (FDA). IDO in combination with temozolomide has shown promise in experimental studies. Dendritic cell vaccines are composed of dendritic cells generated in vitro and then loaded with tumor antigen developed from tumor lysate or cells cultured from surgical specimens. At present, only 1 vaccine (sipuleucel-T) has been approved by the FDA. Other immunotherapeutic options include cytokine therapy, adaptive cell therapy, and oncolytic viruses derived from strains of the herpes simplex virus, polio, and adenoviruses are under investigation. Large-scale population studies for assessing the efficacy of immunotherapy are ongoing.
Response Assessment
MR imaging is essential for assessing treatment response and guiding management. The timing of serial imaging after the baseline pretreatment examination is important to assess structural changes in the brain. However, the temporal evolution of the changes is important and is taken into context relative to the patient’s clinical status. The methodology used to measure response has evolved, but all of the following criteria are modifications from the WHO response criteria reported in 1981, which measured tumors in 2 dimensions.
The Macdonald criteria
These criteria for glioblastoma treatment assessment in 1990 were established in clinical trials to standardize definitions of the imaging features combined with clinical assessments and the use of corticosteroids. Four criteria are summarized as follows:
- •
Complete response (CR): resolution of all enhancing lesions over 4 weeks without the concomitant appearance of new lesions. Patients needed to show clinical improvement with administration of corticosteroids.
- •
Partial response (PR): 50% or more decrease in the enhancement of all measurable lesions over 4 weeks without concomitant appearance of new lesions in patients and continued stability or improvement on reduced dosage of steroids.
- •
Stable disease (SD): clinical stability without features suggestive of progression of disease (PD), PR, or CR.
- •
PD: 25% or more increase in the enhancing lesions with or without appearance of new lesions, together with clinical deterioration.
The Macdonald criteria were limited for the following reasons. There was significant interobserver variability in their application. This methodology also did not take into account the difficulty in measuring residual tumors with irregular contours and the presence of new areas of enhancement after resection. Assessment of multifocal disease was also not included in these criteria. In addition, antiangiogenic therapy masks any potential residual enhancing disease. ,
AVAglio criteria
The Avastin In Glioblastoma (AVAglio) study (BO21990) criteria were developed primarily to address the limitations of the Macdonald criteria. In addition to assessing PD, these criteria consider pseudoprogression based on the MR imaging features on T2 and FLAIR sequences. According to this scheme, the index lesions are defined as all measurable contrast-enhancing lesions with clear borders greater than 10 mm in diameter. These criteria also include assessment of small or irregularly shaped enhancing lesions and lesions that do not enhance. Nonindex lesions were recorded in present, absent, or unable-to-assess categories. These categories were then used to fortify the criteria for CR where the nonindex lesions disappeared for more than 4 weeks, for SD when these lesions showed no significant change, and for PD when there was unequivocal increase in the size of the nonindex lesion or presence of a new nonindex lesion.
Response assessment in Neuro-oncology criteria
Given that the Macdonald criteria were developed for assessment of only glioblastomas and relied on two-dimensional (2D) measurements and the administration of corticosteroids, it was increasing evident that the criteria were deficient in the assessment of transient increase in tumor enhancement (pseudoprogression), development of nonenhancing tumor during antiangiogenic treatment, and the changes in the enhancing characteristics during such therapy (pseudoresponse). This realization led to the development of the Response Assessment in Neuro-oncology (RANO) criteria in 2010 and their modification in 2017. The modified RANO criteria rely on specific MR imaging sequences to assess for treatment response. These sequences include three-dimensional (3D) T1-weighted precontrast, axial 2D FLAIR, axial 2D diffusion-weighted imaging (DWI), axial 2D T2-weighted imaging, and 3D T1-weighted postcontrast sequences. Lesions were classified as measurable and nonmeasurable. Measurable lesions are those with contrast enhancement, with clear margins, that are visible, and that are measurable in 2 or more axial sections. Measurements are performed in 2 perpendicular planes, each more than or equal to 10 mm in sections taken with interslice gaps of less than 5 mm. In those sequences where an interslice gap is greater than 5 mm, the minimum size of the lesions for both perpendicular measurements should be twice the sum of the slice thickness and interslice gap. Up to 5 target measurable lesions should be defined and ranked from largest to the smallest. Nonmeasurable lesions are those that do not meet the criteria of the measurable lesions, do not enhance, or lesions with poorly defined margins that cannot be measured with any degree of certainty.
Similar to the Macdonald criteria, the RANO criteria divide response into CR, PR, PD, and SD while incorporating additional features, as follows:
- •
CR: disappearance of all measurable and nonmeasurable lesions for at least 4 weeks. The patients are required to be off corticosteroids and should be clinically stable or improved since their baseline. The first MR study that shows these feature is termed the preliminary CR. Measurable lesions on any succeeding MR studies indicating unsustained response are termed preliminary PD (progression) or pseudoresponse. Durable CR is identified when the second scan shows continued absence of measurable lesions. Patients showing nonmeasurable lesions at baseline are best categorized as having SD.
- •
PR: defined as 50% or more decrease in measurable lesion, sustained for 4 weeks or more, with no progression of nonmeasurable lesions, and stable or improved nonenhancing FLAIR and T2 signals. Patients should be clinically stable, on reduced or unchanged dosage since baseline. No new lesions should be shown on follow-up studies.
- •
PD: defined as 25% or more increase in the product of perpendicular measurements or 40% increase in volume of the enhancing lesions in 2 successive scans. Measurements are compared with the baseline scan showing the smallest tumor measurement. This baseline study is termed the preliminary PD, and the first study that categorically shows the increase described earlier is termed the confirmed PD study. These criteria are applied 12 weeks or more after completion of radiotherapy. PD is also called when there are new lesions with clinical deterioration that cannot be attributed to radiation, ischemic injury, postoperative sequelae, demyelination, or infections. The findings also should not be attributable to changes in corticosteroid dosage, adverse effects of medications, or seizures. Progression of nonmeasurable lesions is another criterion for PD.
- •
SD requires the patient to be clinically stable on a reduced or unchanged dose of steroids and without imaging criteria on the MR studies that qualify for other categories, such as CR, PR, or PD.
Immunotherapy response assessment for neuro-oncology criteria
With the advent of immunotherapy, the observed changes seen on imaging studies required reassessment of the criteria. Therapy-induced changes showing increased dimensions of the lesions were deemed equivalent to PD. However, these patients remained clinically stable. Because this observed pseudoprogression was not similar to responses seen on standardized chemotherapies (Stupp protocol), patients were taken off these immunotherapy protocols. Modifications to the RANO criteria, Immunotherapy RANO (iRANO), take into account the adverse effects of immunotherapy and the occurrence of new enhancing lesions outside the radiation field. Clinical improvement on immunotherapy can be delayed, and repeat studies are needed to confirm stability or PD. Ideally, studies should be repeated 3 months or later after initiation of therapy as a baseline investigation. The principal diagnostic features of PD after immunotherapy include clinical deterioration caused by the primary tumor alone, or significant changes in measurable lesions after 6 months or more. If measurable changes are observed in a period less than 6 months after initiation of therapy, imaging at 3-month intervals is required to rule out pseudoprogression.
Pseudoprogression presumably occurs as a result of an immune-mediated inflammatory response or PD before treatment can take effect. In these cases, immunotherapy is not interrupted because of changes in lesion size provided that the patient does not show toxicity related to the ongoing therapeutic agent.
Pseudoprogression and Assessment of Response to Combined Radiation and Alkylating Therapy
Pseudoprogression is identified radiographically when tumors undergo growth similar to true PD defined by RANO criteria but occurring between 3 and 6 months’ after treatment, typically with concurrent radiation treatment and temozolomide. Unlike PD, pseudoprogression resolves spontaneously or improves without additional treatment. The incidence of pseudoprogression is reportedly around 36%, making it a frequent occurrence in the treatment arc of gliomas. Pseudoprogression is thought to result from endothelial cell injury resulting from inflammation and upregulation of VEGF, leading on to increasing edema and vascular permeability. Pseudoprogression is seen across the spectrum of low-grade and high-grade gliomas and has clinical implications in that manifestation of pseudoprogression is associated with better outcomes, as is the presence of the O 6 -methylguanine-DNAmethlytransferase (MGMT) promotor methylation. The challenge of differentiating true tumor progression from pseudoprogression remains unresolved.
Advanced imaging techniques
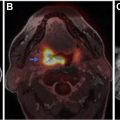
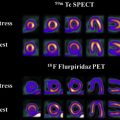
Advanced imaging techniques can play an important role in the differentiation of true tumor progression from pseudoprogression ( Figs. 1 and 2 ). The temporal profile of treatment must be known to more accurately predict whether imaging changes represent PD versus pseudoprogression. The latter typically occurs within the first 3 months after completion of radiation treatment but has a range from a week to up to 6 months after treatment.