High-quality aortic imaging plays a central role in the management of patients with thoracic aortic aneurysm. Computed tomography angiography and magnetic resonance angiography are the most commonly used techniques for thoracic aortic aneurysm diagnosis and imaging surveillance, with each having unique strengths and limitations that should be weighed when deciding patient-specific applications. To ensure optimal patient care, imagers must be familiar with potential sources of artifact and measurement error, and dedicate effort to ensure high-quality and reproducible aortic measurements are generated. This review summarizes the imaging evaluation and underlying pathology relevant to the diagnosis of thoracic aortic aneurysm.
Key points
- •
Cross-sectional imaging (CTA and MRA) plays a central role in management of patients with thoracic aortic aneurysm.
- •
Maximal aortic diameter is the primary metric used to estimate risk and determine the need for surgical repair, although diameter measurement are subject to error related to image artifact and measurement technique.
- •
Optimal imaging surveillance requires selection of imaging modality (CTA vs MRA) based on patient-specific characteristics and indications, in addition to consistent measurement protocols based on double-oblique images to minimize measurement error.
- •
Most TAAs are classified as degenerative and associated with fusiform dilation of the ascending aorta, whereas root aneurysms are typically seen in aortic-related connective tissue disorders and descending thoracoabdominal aneurysms are strongly associated with atherosclerosis.
Introduction
Thoracic aortic aneurysm (TAA) is a chronic condition that manifests as progressive dilation of the thoracic aorta resulting from degradation of the normal smooth muscle cells and extracellular matrix proteins that provide integrity to the aortic wall. TAA is broadly classified into three categories based on cause: (1) degenerative, (2) genetically mediated, and (3) inflammatory (ie, aortitis). Degenerative aneurysms are the most common; are associated with advanced age; occur in the absence of a defined genetic aortopathy or familial clustering; and are associated with cardiovascular risk-factors, such as atherosclerosis and hypertension. Genetically mediated TAAs are those that occur in the setting of a known clinical syndrome (eg, Marfan, Ehlers-Danlos) or in the setting of a genetic mutation in molecular pathways known to be associated with TAA (eg, transforming growth factor-β signaling pathway).
The prevalence of TAA has increased from 3.5 to 7.6 per 100,000 persons between 2002 and 2014. In part, this is caused by increasing rates of incidental detection on unrelated imaging studies (eg, lung cancer screening, coronary computed tomography angiography [CTA]/calcium scoring). Incidental aortic dilation (>4.0 cm) is present in about 3% of patients greater than 55 years old. Maximal aortic diameter is currently the primary metric used to guide surveillance strategy and timing of surgical intervention for patients with TAA. As aortic diameter increases so does the risk of developing life-threatening complications, the most common of which is aortic dissection (ie, delamination of the aortic wall) and less commonly rupture (ie, transmural tearing). In the absence of acute complications, TAAs grow slowly over years or even decades, with typical growth rates in the range of 1 to 3 mm/y. When the aorta size reaches its biomechanical “hinge point,” usually about 6 cm in diameter, wall integrity rapidly declines, growth accelerates, and the incidence of complications rapidly increases. Current guidelines recommend surgical repair of the ascending aorta before the maximal diameter “hinge point” is reached, typically at a threshold of 5.5 cm. The primary management objective for TAA is to identify aortic growth early and to surgically replace the aorta before it reaches a high-risk size.
Noninvasive imaging surveillance plays a central role in the management of TAA through its ability to determine maximal aneurysm diameter and monitor for growth and other complications. Transthoracic echocardiography is used to monitor TAA that is limited to the root and proximal ascending aorta; however, CTA and magnetic resonance angiography (MRA) are the most common imaging modalities for evaluation of TAA because they can evaluate the entire thoracic aorta without the limitations of acoustic windows. Current guidelines generally lack detailed recommendations for the frequency of imaging surveillance and there are variations in approaches between physicians and centers; however, it is generally agreed that in degenerative TAA where the degree of dilation is mild or moderate (4.0–5.0 cm), annual follow-up imaging is appropriate with spacing to biennial or triennial if aortic dimensions have shown long-term stability. , When aortic dimensions are clearly increasing or approaching surgical thresholds, imaging frequency is typically increased to biannual.
Normal aortic anatomy
The thoracic aorta is divided into the following regions: aortic root, ascending aorta, aortic arch, and descending aorta. The aortic root includes the annulus, aortic valve, and sinuses of Valsalva. The conventional aortic anatomy consists of three sinuses corresponding to the aortic valve cusps (right, left, and noncoronary). The three sinuses of Valsalva taper and form a “waist” at their junction with the tubular ascending segment (ie, the sinotubular junction [STJ]). The tubular ascending aorta extends from the STJ to the first arch vessel, and is so named given its lack of branches and resemblance to simple “tube.” Beyond the tubular segment, the aorta arch gives rise to the arch vessels (innominate, left common carotid, and left subclavian) from the proximal aortic arch. The distal arch beyond the left subclavian artery to the region of the ligamentum arteriosum is called the aortic isthmus. This region is of clinical significance, because it is a common site of nonfatal traumatic aortic injury and coarctation. The descending thoracic aorta extends to the diaphragmatic hiatus. Guidelines suggest that aortic diameters be reported at specific aortic locations along the aortic length including the sinuses of Valsalva, STJ, midascending aorta, proximal and distal arch, middescending aorta, and at the diaphragmatic hiatus. Either sinus-to-sinus or sinus-to-commissure measurements may be reported for the sinuses of Valsalva.
Normal sizes for the thoracic aorta have been defined from several reference populations. In general, aortic size increases with patient age, male gender, and body size. , However, measurement techniques can introduce variability into the reported size of the thoracic aorta. These include measuring the aorta using gated versus nongated imaging technique (and when gated, during systole vs diastole), from inner versus outer edge, and in the axial versus double-oblique planes. The next section explores best practices of measurement technique.
The range of mean ascending aortic diameters (including gated and nongated examinations) in the literature by computed tomography (CT) ranges from 29.0 to 37.2 mm for females, and 30.8 to 39.1 mm for males, with the larger diameters reported for studies without electrocardiographic (ECG)-gating. Although in general it is accepted that the maximal diameter of the ascending thoracic aorta should be lower than 40 mm in healthy individuals, some series have shown that the normal range (within two standard deviations of the mean) for males and females can extend above this level. Considering the significant impact of patient size on normal aortic diameter, indexing aortic dimensions to adjust for patient body size (ie, height or body surface area) is appropriate for optimal definition of pathologic aortic dilation; however, clinical application of indexed aortic measurements in adults is limited because of the lack of comprehensive population nomograms to determine reference ranges.
Imaging technique
When selecting an imaging technique, the strengths and weaknesses of various imaging modalities should be considered in relation to the clinical context. The American College of Radiology Appropriateness Criteria for TAA initial imaging rates CTA and MRA as “usually appropriate.” For preprocedure planning before thoracic endovascular repair (TEVAR), CTA chest, abdomen, and pelvis is rated at 9 “usually appropriate,” whereas MRA and CTA chest alone are rated at 7 “usually appropriate.” CTA is often preferable to MRA following TEVAR given the increased artifact as a result of metal stent (particularly those composed of stainless steel) and the increased ability of CTA to detect postoperative infection and endoleak. Pros and cons of CTA versus MRA are summarized in Table 1 .
| Pros and Cons of CTA vs MRA | ||
|---|---|---|
| Characteristic | CTA | MRA |
| Radiation | Ionizing radiation (x-ray) + DNA damage | Nonionizing (radiofrequency) No DNA damage |
| Spatial resolution (typical) | 0.5–1.5 mm 3 | 0.7–1.5 mm 3 (variable) |
| Number of acquisitions | Usually single | Usually multiple |
| Set-up and scan time | Short (5–10 min) | Long (45–60 min) |
| Acquisition complexity | Easy | More difficult |
| Patient participation | Minimal | Significant: multiple breath hold |
| Modality strength | Anatomy and postsurgical evaluation | Soft tissue characterization and hemodynamic/functional assessment |
| Contrast risk |
|
|
Measurement Techniques
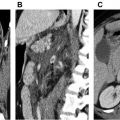
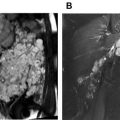
Measurement techniques can introduce significant variability into the reported size of the thoracic aorta. Different measurement techniques used in clinical practice by different centers have been shown to result in a lower reproductivity for CT compared with echocardiography. One method to reduce this variability is through the use of double-oblique or orthogonal measurements. Double-oblique measurement obtained orthogonal to the aortic centerline allows creation of a true short axis reformation of the aortic diameter and has been shown to allow more accurate measurement of aortic size compared with axial measurement ( Fig. 1 ). Axial measurement may result in a significant overestimation of aortic size, up to 6 mm or 21% increase in size according to Hager and colleagues. In one series, axial measurements were shown to overestimate aortic size at multiple locations (with the exception of the aortic arch) and resulted in the misclassification of 13% of patients into either aneurysmal or surgical candidate categories ( Fig. 2 ). It is also important to recognize that different measurement approaches at the aortic wall such as inner to inner, leading edge, or outer to outer can also introduce variation in aortic diameter. Consensus as to which of these methods is preferred has not been established for CT and MR imaging, although leading edge to leading edge is a frequent standard used with echocardiography. Within a center, consistent technique should be adopted to decrease measurement variability between serial scans.


Imaging protocols
The thoracic aorta is best evaluated with cross-sectional imaging, either CT or MR imaging. Although CTA and MRA imaging techniques are routinely used to evaluate the aortic size and structure, specific CT and MR imaging protocols are additive in evaluating thoracic aortic pathology.
Computed Tomography
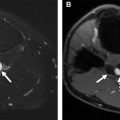
The standard multidetector CT evaluation of TAA consists of contrast-enhanced CTA. Noncontrast CT may be obtained before CTA to assess for intramural hematoma (IMH) in the setting of concern for acute aortic syndrome or to assess for calcification or surgical material in a postoperative patient. It is important to distinguish aortic wall thickening resulting from atherosclerosis, which presents as circumferential aortic wall thickening that is stable over time, from acute IMH, which tends to be eccentric in location and hyperdense of non-contrast series ( Fig. 3 ). Contrast-enhanced CTA of the aorta may be performed with bolus tracking or use of a timing bolus to ensure optimal enhancement of the thoracic aorta. Postcontrast delayed phase images may also be obtained in patients with endovascular repair of TAA or dissection (TEVAR) to assess for endoleak or in patients with inflammatory TAA/aortitis to evaluate for periadventitial enhancement indicative of active inflammation.