Magnetic resonance (MR)/PET scanners provide an imaging platform that enables simultaneous acquisition of MR and PET data in perfect spatial and temporal registration. This feature allows improving image quality for the MR and PET images obtained during the course of an examination. In this work the authors demonstrate the use of prospective MR-based motion tracking information for removing motion blur in MR/PET images of small pulmonary nodules. The theoretical basis for the algorithms is presented alongside clinical examples of its use.
Key points
- •
Assessment of small lung nodules is challenging because of the significant blur due to respiratory (and often nonperiodic motion).
- •
Tracking respiratory motion during PET/computed tomography and MR/PET imaging using external devices is limited because of the small dynamic range of the devices and the inherent assumption that the respiratory motion is periodic throughout the examination.
- •
Prospective respiratory motion tracking and correction does not require the motion to be periodic and, as a result, leads to improved quantitative assessment of standard uptake values for small pulmonary nodules.
Introduction
The development of the MR/PET imaging platform provides, for the first time, a true synergistic imaging platform whereby information from each of the component modalities can be used to improve their individual image quality. Examples would be the use of high-resolution MR imaging scans to improve the quality of the PET images or the use of PET images to reject undersampling artifacts from highly undersampled dynamic MR imaging scans. The installed base of MR/PET systems is currently relatively small. However, the complementary nature of the information provided by the two modalities together with the synergisms implicit in collecting simultaneous information have allowed this platform to become the preferred choice for the diagnosis of conditions, such as dementia, neurofibromatosis, and epilepsy. For oncologic imaging, there are several applications whereby the MR/PET technology is poised to make significant improvements because of its improved workflow and capabilities. One of the oncologic applications that might benefit greatly from the capabilities of a simultaneous MR/PET acquisition is lung cancer. Lung cancer is the leading cause of cancer-related death in the Western world. Early detection of lung cancer is challenging, as the symptoms are often very similar to those of other common respiratory ailments. Multidetector computed tomography (MDCT) is routinely used for evaluation of disease in the lung and can be routinely performed without intravenous contrast administration and with a low radiation dose. Although MDCT has high sensitivity for nodule detection, it has limited specificity in discriminating malignant from benign nodules even in setting of primary malignancy. Assessment of metabolic activity within lung nodules with fludeoxyglucose F18 (18F-FDG) PET is often improved when compared with computed tomography (CT) for discriminating benign from malignant nodules, making PET/CT the preferred modality to assess whole-body metastatic burden in many primary malignancies.
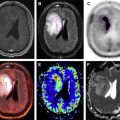
However, there are concerns with respect to the accuracy of PET/CT in the detection and characterization of subcentimeter pulmonary. Specifically, nodules of less than 1 cm in diameter have a high (40%) false-negative rate. The high false-negative rate of FDG PET for small lesions seems to be related to respiratory and cardiac motion and the sequential nature for the acquisition of the PET and CT images when using a conventional PET/CT scanner. Studies have demonstrated mild improvements of standardized uptake value (SUV) quantification in PET/CT of pulmonary nodules when respiratory motion correction, via external monitoring devices, is used. Thus, motion-corrected PET reconstruction can potentially improve detection and characterization of pulmonary nodules.
Hybrid PET/MR systems allow for simultaneous acquisition of PET and MR imaging. It has been shown that PET acquisition performed simultaneously with free-breathing radial T1-weighted MR acquisition has similar accuracy as PET/CT for FDG-avid lesions and lesions larger than 5 mm in size. However, despite simultaneous acquisition of PET and MR, hybrid PET/MR has had low sensitivity for small pulmonary nodules that did not show FDG avidity. Thus, similar to PET/CT, conventional (without motion correction) simultaneous PET/MR remains limited for detection and characterization of small pulmonary nodules because of its inability to confidently detect FDG uptake in small lesions, which is likely related to motion blur and respiratory motion.
Introduction
The development of the MR/PET imaging platform provides, for the first time, a true synergistic imaging platform whereby information from each of the component modalities can be used to improve their individual image quality. Examples would be the use of high-resolution MR imaging scans to improve the quality of the PET images or the use of PET images to reject undersampling artifacts from highly undersampled dynamic MR imaging scans. The installed base of MR/PET systems is currently relatively small. However, the complementary nature of the information provided by the two modalities together with the synergisms implicit in collecting simultaneous information have allowed this platform to become the preferred choice for the diagnosis of conditions, such as dementia, neurofibromatosis, and epilepsy. For oncologic imaging, there are several applications whereby the MR/PET technology is poised to make significant improvements because of its improved workflow and capabilities. One of the oncologic applications that might benefit greatly from the capabilities of a simultaneous MR/PET acquisition is lung cancer. Lung cancer is the leading cause of cancer-related death in the Western world. Early detection of lung cancer is challenging, as the symptoms are often very similar to those of other common respiratory ailments. Multidetector computed tomography (MDCT) is routinely used for evaluation of disease in the lung and can be routinely performed without intravenous contrast administration and with a low radiation dose. Although MDCT has high sensitivity for nodule detection, it has limited specificity in discriminating malignant from benign nodules even in setting of primary malignancy. Assessment of metabolic activity within lung nodules with fludeoxyglucose F18 (18F-FDG) PET is often improved when compared with computed tomography (CT) for discriminating benign from malignant nodules, making PET/CT the preferred modality to assess whole-body metastatic burden in many primary malignancies.
However, there are concerns with respect to the accuracy of PET/CT in the detection and characterization of subcentimeter pulmonary. Specifically, nodules of less than 1 cm in diameter have a high (40%) false-negative rate. The high false-negative rate of FDG PET for small lesions seems to be related to respiratory and cardiac motion and the sequential nature for the acquisition of the PET and CT images when using a conventional PET/CT scanner. Studies have demonstrated mild improvements of standardized uptake value (SUV) quantification in PET/CT of pulmonary nodules when respiratory motion correction, via external monitoring devices, is used. Thus, motion-corrected PET reconstruction can potentially improve detection and characterization of pulmonary nodules.
Hybrid PET/MR systems allow for simultaneous acquisition of PET and MR imaging. It has been shown that PET acquisition performed simultaneously with free-breathing radial T1-weighted MR acquisition has similar accuracy as PET/CT for FDG-avid lesions and lesions larger than 5 mm in size. However, despite simultaneous acquisition of PET and MR, hybrid PET/MR has had low sensitivity for small pulmonary nodules that did not show FDG avidity. Thus, similar to PET/CT, conventional (without motion correction) simultaneous PET/MR remains limited for detection and characterization of small pulmonary nodules because of its inability to confidently detect FDG uptake in small lesions, which is likely related to motion blur and respiratory motion.
Lung motion correction in magnetic resonance/PET: previous limitations
Abdominal motion is still an outstanding concern for PET image reconstruction, particularly in the abdomen where rigid body approximations do not apply. Most of the motion correction algorithms previously used for PET image reconstruction rely on the use of retrospective corrections whereby the data are grouped according to the time of acquisition relative to the phase of the respiratory cycle (or any other motion of interest) as assessed by an additional motion tracking device (usually a respiratory belt/cushion). In this setting, different segments from the list-mode data set, corresponding to different data acquisition times, will often be grouped together, regardless of whether the respiratory motion is periodic and/or the physiologic process stationary. As a result, the performance of such algorithms is highly variable as it depends strongly on the reproducibility of the respiratory motion throughout the examination. Unfortunately, studies have demonstrated that breathing motion varies considerably for healthy human controls and much more so for those individuals whereby lesion is present. Consequently, respiratory motion correction via external tracking devices has limited effectiveness and continues to be an area of active research in PET image reconstruction. As a result, there is no standard for dealing with this challenge and the available methods are not often part of the clinical workflow for PET/CT and MR/PET examinations.
Retrospective Motion Correction for Magnetic Resonance/PET Data
Motion correction algorithms for MR/PET data were introduced shortly after the first system prototypes were delivered. In the setting of MR/PET, the use of a simultaneous acquisition mode, whereby both MR and PET data are acquired at the same time, allows tracking the respiratory motion using MR signals (images or raw data). Use of the MR-based tracking information can be done prospectively and retrospectively. Retrospective approaches are, conceptually, very similar to those previously used for PET/CT, whereby the data are, again, retrospectively reordered. Therefore, these algorithms are easy to implement but have limited effectiveness because they still suffer from some of the limitations stated earlier.
Prospective Motion Correction for Magnetic Resonance/PET Data
Prospective motion correction algorithms rely on continuous information about the abdomen’s deformation for the generation of motion fields that can then be used to dynamically distort the system matrix so that all the list-mode data can be intrinsically coregistered to a single-motion state or gate. As such, they do not require the respiratory motion to be periodic and can also be used during dynamic studies by simply binning the list-mode data for physiologically appropriate time windows. This approach is conceptually different from the methodology presented elsewhere in this volume. A brief description of its fundamental elements is, therefore, presented later.
In order to obtain a single-motion free PET reconstruction using this approach, the conventional straight-line model is extended by a motion component in which respiratory motion information is integrated within the PET system matrix. To achieve this, the expectation maximization algorithm
is modified to
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree