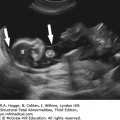
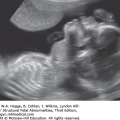
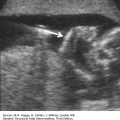
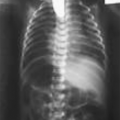
Definition Cytomegalovirus (CMV) is a large, enveloped DNA herpesvirus. Adult infection is frequently asymptomatic, but fetal infection may cause severe damage to multiple organ systems. CMV is transmitted in adults by bodily secretions. Fetal exposure occurs transplacentally.
Epidemiology CMV is the most common cause of in utero infection; approximately 0.5% to 1% of pregnant women are infected. Maternal CMV infection usually presents with few, if any, side effects. When first infected, some people may have symptoms similar to mononucleosis (i.e., fatigue, weakness, fever, lymphadenopathy). Most people in the United States are infected during childhood or as adults if they work around children. Pregnant women who have not been infected with CMV in the past and become infected during pregnancy (i.e., a primary infection) are at particularly increased risk of fetal CMV infection. Of note, recurrent or reactivation CMV infection in women can also cause fetal CMV infection, although the likelihood and severity of fetal infection is lower and less severe than with primary maternal CMV infection. For pregnant women, the two most common exposures to CMV are through sexual contact and through contact with the urine of young children with CMV infection. Most CMV infections encountered in pregnant women are asymptomatic, even during the acute stage. Less than 5% of pregnant women with primary infection are reported to be symptomatic, and an even smaller percentage suffer from a mononucleosis syndrome
Prognosis The severity of disease and its manifestations are determined by the timing of infection and the affinity of nervous tissue to different CMV strains. A normal second-trimester scan does not exclude an abnormal ultrasound examination later in gestation.
Hepatic calcifications
Splenic calcifications
Hydrops
Pleural effusions
Ascites
Anasarca
Scalp edema
Placentomegaly
Cardiomegaly
Ventriculomegaly
Central nervous system
Microcephaly
Periventricular echogenic halo, indicating white matter necrosis
Linear calcifications in the basal ganglia
Punctate cerebellar calcifications
Intraventricular hemorrhage
Porencephaly
Lissencephaly
Subependymal cysts
Microphthalmia
Echogenic small bowel
Hepatomegaly
Splenomegaly
Punctate calcifications
Pericardial
Placental
Echogenic nephropathy
Investigations and Consultations Required Maternal blood serologic testing is helpful in delineating the acuity of CMV infection. Serologic testing for CMV may be considered for women who develop influenza-like illness or a viral hepatitis-like illness during pregnancy or following detection of sonographic findings suggestive of CMV infection. Maternal blood ought to be tested for CMV immunoglobulin (Ig) M, IgG, and IgG avidity. To ascertain if fetal infection has occurred, amniocentesis can be performed, with testing of amniotic fluid for CMV-specific polymerase chain reaction (PCR). Amniocentesis should be timed for at least 7 weeks after the presumed time of maternal infection and at 21 weeks or more of gestation. This interval is important because it takes 5 to 7 weeks following fetal infection, and subsequent replication of the virus in the kidney, for a detectable quantity of the virus to be secreted to the amniotic fluid.
If CMV is suspected, diagnosed because of fetal ultrasound findings, or the fetus is symptomatic, a more aggressive approach is warranted. An extended ultrasound should be performed. Consultations with maternal fetal medicine, neonatology, or infectious disease are useful for counseling parents about prognosis and outlining an approach to pregnancy management and neonatal issues.
Maternal Treatment Currently, there are no licensed treatments for pregnant women who become infected with CMV during pregnancy. Treatments that are effective against CMV infection in the nonpregnant population have serious side effects, are not approved for use in pregnant women, and have not been shown to prevent CMV infection in the fetus. Likewise, treatment with hyperimmune globulin does not significantly modify the course of primary CMV infection during pregnancy.
Fetal Intervention Symptomatic fetuses will not have the course of their infection altered by currently available treatments in utero. Intrauterine transfusion is a successful treatment for the rare case of fetal anemia secondary to CMV infection, although in these cases prognosis is poor with or without transfusion.
Monitoring In the setting of confirmed fetal CMV infection, interval ultrasound examinations should be performed to measure growth and exclude the development of sequelae of infection in the fetus, particularly in the central nervous system. In addition, the fetus can be monitored for the development of hydrops. These findings confer a worsening prognosis and warrant reconsultation with the parents and pediatric subspecialists and transfer to a tertiary center for delivery.
Pregnancy Course Most women with CMV are asymptomatic. Among those with influenza-like or hepatitis-like illness, supportive care ought to be given. Pregnancy course otherwise is unaltered.
Pregnancy Termination Issues If pregnancy termination is undertaken, fetal and placental tissues should be tested for CMV by PCR and have histopathologic examination.
Delivery The mode of delivery should be based on obstetric indications and will vary depending on the condition of the fetus. Delivery should be at a tertiary center for symptomatic fetuses.
Resuscitation In the absence of respiratory distress, significant hydrops, or severe anemia, resuscitative efforts are usually not required. Premature infants born with congenital CMV are more likely to present with pneumonitis and may require respiratory support
Transport In symptomatic infants, transfer to a tertiary center with neonatology and pediatric infectious disease subspecialists is indicated. Infants with hydrops or CMV pneumonitis may require ventilatory assistance in transit.
Testing and Confirmation Laboratory diagnosis of congenital CMV infection is accomplished by isolation or molecular detection of CMV from urine or saliva samples collected within the first 3 weeks of life. Both viral culture and PCR tests have high sensitivity and specificity for detection of CMV in infected neonates.
Nursery Management Although infants born prematurely or with severe disease at birth have an increased risk of morbidity and mortality, most infants with congenital CMV infection are asymptomatic. Approximately 10% of infected neonates have symptoms at birth. Clinical findings may include petechiae, jaundice, hepatosplenomegaly, small for gestational age, and microcephaly. In symptomatic infants with congenital CMV, common laboratory findings include thrombocytopenia, hemolytic anemia, elevated transaminases, and conjugated hyperbilirubinemia. Abdominal ultrasound may demonstrate intrahepatic calcifications. Findings on cranial imaging (magnetic resonance imaging [MRI], head ultrasound, or computed tomographic [CT] scan) include periventricular intracranial calcifications, ventriculomegaly, lenticulostriate vasculopathy, migrational abnormalities, or periventricular leukomalacia. Chorioretinitis is the most common ophthalmologic abnormality in symptomatic infants. Abnormalities on cranial imaging or the presence of chorioretinitis correlates with poor neurodevelopmental outcome.
Congenital CMV is the leading cause of sensorineural hearing loss in infants. Sensorineural hearing loss occurs in 33% to 50% of symptomatic infants. Infants with symptomatic congenital CMV require routine serial hearing screens, as the sensorineural hearing loss may be progressive.
Antiviral treatment with ganciclovir and valganciclovir is recommended for symptomatic infants with virologically proven CMV infection and at least one end-organ symptom. Antiviral treatment in symptomatic infants has been shown to improve both long-term audiologic and neurodevelopmental outcomes.
Definition Parvovirus B19 is a small, nonenveloped DNA virus that exclusively infects humans. After infection, parvovirus B19 replication occurs primarily in erythrocytes and erythroblasts, which can lead to anemia in predisposed individuals. Children who are infected with parvovirus B19 typically develop erythema infectiosum (fifth disease) that is characterized by a “slapped-cheek” rash, low-grade fever, and mild influenza-like symptoms. Infected healthy adults generally have mild constitutional symptoms only. In contrast, immune-compromised individuals, including fetuses, can develop severe chronic anemia requiring directed therapies.
Epidemiology Transmission occurs via respiratory secretions. Although outbreaks may occur sporadically year-round, they are most prevalent in childcare facilities or schools in the spring. Infection transmitted in utero from a susceptible mother to the immune-incompetent fetus is recognized as an infrequent cause of fetal morbidity and mortality. Risk factors for maternal infection include large number of children in the household and occupational exposure (such as teacher or day-care worker). Fetal parvovirus B19 infection can cause spontaneous abortion in the early part of pregnancy and nonimmune hydrops fetalis secondary to aplastic anemia later in pregnancy.
Prognosis There is a 10% risk of fetal death due to hydrops secondary to severe fetal anemia. The risk of fetal death in utero is 17% before 20 weeks’ gestation and 6% after 20 weeks. Most infected women give birth to normal neonates.
Hydrops
Pleural effusion
Pericardial effusion
Anasarca
Scalp edema
Placentomegaly
Polyhydramnios
Cardiomegaly
Ventriculomegaly
Microcephaly
Hepatosplenomegaly
Hepatic calcifications
Echogenic small bowel
The peak systolic velocity in the middle cerebral artery is elevated with severe anemia.
The risk of hydrops decreases from 5% to 10% in the first trimester to 1% at 20 weeks’ gestation.
Hydrops develops between 3 and 12 weeks after maternal infection.
Most cases of hydrops occur between 16 and 32 weeks’ gestation.
For women who report an exposure to an individual with parvovirus B19, we suggest following the screening and surveillance algorithm as outlined in the figure that follows.
Investigations and Consultations Required Patients who are noted to have serologic confirmation of recent infection should be referred to a perinatal center that is equipped to perform sonographic surveillance for fetal anemia with middle cerebral artery peak systolic velocity.
Maternal Treatment There are no applicable antiviral therapies, vaccines, or immunological therapies during pregnancy or in the neonatal period.
Monitoring The serologic and sonographic surveillance strategy, as outlined in the figure, is recommended for monitoring.
Fetal Intervention Intrauterine transfusion is a successful treatment for fetal anemia secondary to parvovirus infection. Serial transfusions may be necessary, but as the infection is self-limited, they will not be necessary throughout the remainder of the pregnancy. Once the anemia has resolved with or without the need for transfusion, interval follow-up ultrasound examinations to monitor fetal growth and possible sequelae of the infection are warranted.
Pregnancy Course For women with maternal symptoms related to parvovirus infection, supportive care is reasonable, with antipyretics and analgesics for symptomatic relief. In pregnancies with an infected fetus, once the anemia is treated with transfusions, no other pregnancy complications are expected.
Pregnancy Termination Issues If pregnancy termination is undertaken, fetal and placental tissues should be tested for parvovirus B19 by PCR and histopathologic examination.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree