Chapter Outline
Human Immunodeficiency Virus Esophagitis
Because of the increased survival of immunocompromised patients with malignant neoplasms, organ transplants, and other debilitating diseases, infectious esophagitis has become an increasingly common problem in modern medical practice. Candida albicans is the usual offending organism, but herpes simplex virus and cytomegalovirus (CMV) have also been recognized with increased frequency as opportunistic esophageal invaders. Patients with AIDS may develop more fulminant forms of fungal and viral esophagitis (including human immunodeficiency virus [HIV] esophagitis), accentuating the need for early diagnosis and treatment.
Candida Esophagitis
Pathogenesis
Candidiasis is the most common cause of infectious esophagitis. C . albicans is almost always the offending organism. Because C . albicans is a commensal inhabitant of the pharynx, Candida esophagitis is presumably caused by downward spread of the fungus to the esophagus. Clinically significant infection occurs primarily in patients who are immunocompromised because of underlying malignant tumor, debilitating illness, diabetes, or treatment with radiation, steroids, or other cytotoxic agents. Candida esophagitis is particularly prevalent in patients with AIDS, occurring in 15% to 20% of these individuals, although effective antiviral agents have substantially decreased the number of HIV-positive patients who develop AIDS.
Local esophageal stasis is another factor that predisposes patients to the development of Candida esophagitis. Esophageal stasis may be caused by mechanical obstruction from achalasia or strictures or by physiologic obstruction from scleroderma or other causes of esophageal aperistalsis. Delayed esophageal emptying in these patients permits the fungal organism to overgrow and colonize the esophagus with subsequent esophagitis.
Much less frequently, Candida esophagitis may develop in otherwise healthy individuals who have no underlying systemic or esophageal diseases. As a result, the possibility of fungal infection should not be excluded simply because the classic predisposing factors are not present in a particular patient.
Clinical Findings
Most patients with Candida esophagitis have acute onset of dysphagia or, even more commonly, odynophagia, characterized by intense substernal pain during swallowing. Others may have nonspecific findings such as chest pain, epigastric pain, or upper gastrointestinal bleeding, or they may be asymptomatic. Occasionally, patients with chronic Candida esophagitis may have persistent dysphagia because of the development of esophageal strictures.
Despite the characteristic presentation, Candida esophagitis may be difficult to differentiate from viral esophagitis on clinical grounds. The presence of oropharyngeal candidiasis (i.e., thrush) is a helpful finding, but only 50% to 75% of patients with Candida esophagitis have fungal lesions in the oropharynx. Other patients with thrush may have herpes or CMV esophagitis, so the presence of oropharyngeal candidiasis does not preclude the development of viral esophagitis. Still other patients may have concomitant Candida and herpes esophagitis, most likely resulting from fungal superinfection of herpetic ulcers.
Immunocompromised patients with Candida esophagitis require treatment with potent antifungal agents such as fluconazole. Affected individuals usually have a marked clinical response to antifungal therapy. In one study, however, recurrent Candida esophagitis occurred in 90% of successfully treated AIDS patients.
Endoscopic Findings
Candida esophagitis is usually characterized at endoscopy by patchy, white exudates covering a friable, erythematous mucosa. In more advanced disease, the mucosa becomes ulcerated and necrotic with extensive pseudomembrane formation. The presence of budding yeast cells, hyphae, and pseudohyphae on endoscopic biopsy specimens with silver stain, periodic acid–Schiff stain, or Gram stain is diagnostic of Candida esophagitis.
Radiographic Findings
Candida esophagitis tends to be a superficial disease with mucosal abnormalities that are difficult to recognize on conventional single-contrast barium studies. As a result, single-contrast esophagography has been an unreliable technique for detecting this condition, with a reported sensitivity of less than 50%. In contrast, double-contrast esophagography has a sensitivity of about 90% in diagnosing Candida esophagitis. The major advantage of this technique is its ability to demonstrate mucosal plaques that cannot easily be seen on single-contrast studies.
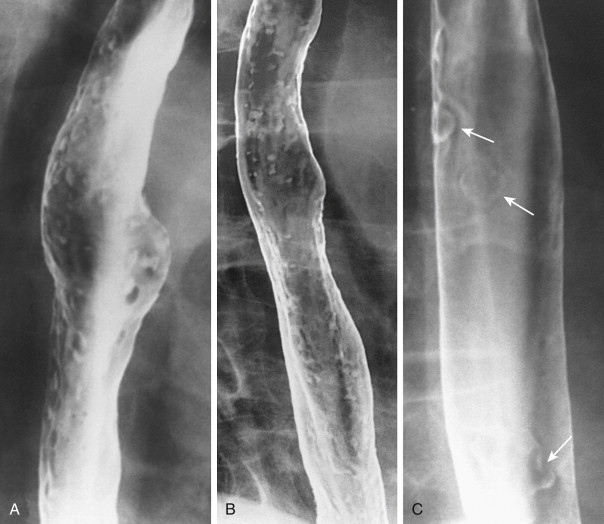
Candida esophagitis is usually manifested on double-contrast images by discrete plaquelike lesions consisting of small exudates and pseudomembranes on the mucosa. The lesions tend to be longitudinally oriented, appearing en face as discrete, linear or irregular filling defects with normal intervening mucosa ( Fig. 20-1 ). The plaques are located predominantly in the upper or midesophagus, occasionally having a focal distribution ( Fig. 20-2 ). In the appropriate clinical setting, discrete plaquelike lesions should be highly suggestive of Candida esophagitis.


In other patients, Candida esophagitis may be manifested by a finely nodular or granular appearance because of tiny plaques on the mucosa ( Fig. 20-3 ). Some plaques may contain central umbilications that collect barium, mimicking the appearance of tiny ulcers caused by herpes esophagitis. When larger plaques are present, the lesions may coalesce, producing a distinctive snakeskin appearance ( Fig. 20-4 ). Occasionally, submucosal edema and inflammation may result in thickened longitudinal folds, a nonspecific manifestation of esophagitis. Thus, the classic radiographic features of Candida esophagitis are not present in all patients.


In severe candidiasis, the esophagus may have a grossly irregular or shaggy contour because of coalescent plaques and pseudomembranes, with trapping of barium between these lesions ( Fig. 20-5 ). Some of these plaques and pseudomembranes may eventually slough, producing one or more deep ulcers superimposed on a background of diffuse plaque formation (see Fig. 20-5B ). This fulminant form of candidiasis has been encountered primarily in patients with AIDS. As a result, the shaggy esophagus of Candida esophagitis has become a less common finding as more effective antiviral medications have become available to prevent the development of AIDS in HIV-positive patients. Nevertheless, the possibility of AIDS should be suspected when a shaggy esophagus is detected on barium studies, particularly in high-risk patients.

Candida esophagitis may occasionally produce other unusual radiographic findings. In some patients, barium may dissect beneath plaques or pseudomembranes, producing an intramural track or double-barreled esophagus. Rarely, a coalescent mass of heaped-up necrotic debris and fungal mycelia (a fungus ball) may be indistinguishable from a polypoid esophageal carcinoma. Esophageal obstruction, perforation, and tracheoesophageal or aortoesophageal fistula formation are other rare but potentially life-threatening complications.
Candida esophagitis usually responds quickly to antifungal therapy, but resolution of the radiographic findings sometimes lags behind the clinical recovery, so follow-up barium studies may still be abnormal in patients who are asymptomatic. The immediate effects of antifungal therapy should therefore be assessed primarily on clinical grounds.
Although Candida esophagitis is usually self-limited with proper treatment, occasional cases of stricture formation have been reported. These strictures typically appear as long, tapered areas of esophageal narrowing ( Fig. 20-6 ). Fungal-induced strictures should be distinguished from pseudostrictures caused by esophageal spasm or the patient’s inability to swallow an adequate bolus of barium. Therefore a second examination may be necessary after treatment to determine if a true stricture is present.

Because of the effects of local esophageal stasis (see earlier, “ Pathogenesis ”), patients with conditions such as achalasia and scleroderma are at increased risk for developing Candida esophagitis. Such cases may be manifested on esophagography by tiny nodular defects, polypoid folds, or a distinctive lacy appearance in the esophagus ( Fig. 20-7 ). Because of esophageal stasis in patients with achalasia or scleroderma, these individuals may also develop a foamy esophagus characterized by innumerable tiny, rounded bubbles that settle out along the top of the barium column, producing a layer of foam ( Fig. 20-8 ). It has been postulated that this finding is caused by extensive production of carbon dioxide by a yeast form of the organism. Whatever the explanation, Candida esophagitis should be suspected when a foamy esophagus is detected on esophagography, particularly in patients with achalasia or scleroderma.


Candida esophagitis is also known to be associated with esophageal intramural pseudodiverticulosis (see Chapter 21 ). It has been postulated that the pseudodiverticula develop as a complication of fungal infection. It is more widely believed, however, that the fungal organism is a secondary invader as a result of local stasis.
Patients with defects in their cell-mediated immune response to C . albicans may have an unusual disease known as chronic mucocutaneous candidiasis, in which there is persistent fungal infection of the skin, mucous membranes, and nails. Although uncommon, esophageal involvement may lead to chronic esophageal candidiasis. In contrast to acute Candida esophagitis, this entity is characterized by chronic scarring and stricture formation in the esophagus. The presence of a long esophageal stricture in patients with chronic mucocutaneous candidiasis should therefore suggest the possibility of esophageal involvement by this disease.
Differential Diagnosis
Mucosal plaques or nodules may also be caused by herpes esophagitis, reflux esophagitis, glycogenic acanthosis, and superficial spreading carcinoma. Although herpes esophagitis is usually manifested by multiple small, discrete ulcers in the esophagus (see later, “ Herpes Esophagitis ”), advanced herpetic infection may lead to the development of plaquelike lesions indistinguishable from those in Candida esophagitis ( Fig. 20-9 ).

Reflux esophagitis may also produce a nodular or granular appearance of the mucosa that resembles candidiasis. However, the nodules of reflux esophagitis tend to have poorly defined borders that fade peripherally into the adjacent mucosa, whereas the plaques of candidiasis have more discrete borders. The nodular mucosa of reflux esophagitis also occurs as a continuous area of disease, extending proximally from the gastroesophageal junction, whereas Candida esophagitis often spares the distal esophagus. Rarely, severe reflux esophagitis may produce inflammatory exudates or pseudomembranes that are indistinguishable on double-contrast studies from the plaquelike lesions of candidiasis.
Glycogenic acanthosis may also be manifested by discrete plaques or nodules, mimicking the appearance of Candida esophagitis. However, the nodules of glycogenic acanthosis tend to have a more rounded appearance, whereas the plaques of candidiasis usually have a more linear configuration. The clinical history is also helpful for differentiating these conditions because patients with glycogenic acanthosis are almost always asymptomatic.
Superficial spreading carcinoma of the esophagus is characterized by focal nodularity of the mucosa that could be mistaken for a localized area of Candida esophagitis. However, candidiasis usually produces discrete plaquelike lesions separated by segments of normal intervening mucosa, whereas the plaques or nodules of superficial spreading carcinoma tend to coalesce, producing a continuous area of disease. Rarely, an advanced infiltrating carcinoma extending longitudinally in the wall can mimic the shaggy esophagus of candidiasis ( Fig. 20-10 ).

Mucosal plaques may be simulated by technical artifacts on double-contrast studies, including air bubbles, debris, and undissolved effervescent agent ( Fig. 20-11 ). When candidiasis is suspected on clinical grounds, double-contrast images of the esophagus should therefore be obtained before administration of an effervescent agent. If the radiographic findings are equivocal, additional double-contrast images may be obtained to demonstrate the transient nature of these artifacts.

Herpes Esophagitis
Pathogenesis
Herpes simplex virus type 1, a DNA core virus, has been recognized as another common cause of infectious esophagitis in patients who are immunocompromised because of underlying malignant tumor, debilitating illness, AIDS, or treatment with irradiation, chemotherapy, or steroids. This infection should be suspected in the same clinical setting as candidiasis. Occasionally, however, herpes esophagitis may occur as an acute, self-limited disease in otherwise healthy individuals who have no underlying immunologic problems. Thus, the diagnosis of herpes esophagitis should not be excluded because the patient has a normal immunologic status.
Clinical Findings
Patients with herpes esophagitis typically present with acute odynophagia, characterized by severe substernal chest pain during swallowing. Other patients may have dysphagia, chest pain and, less commonly, upper gastrointestinal bleed-ing. In the appropriate clinical setting, the presence of herpetic lesions in the oropharynx should suggest a diagnosis of herpes esophagitis. Most patients do not have active infection of the oropharynx, however, so the absence of oropharyngeal lesions does not preclude this diagnosis. Furthermore, some patients with herpetic lesions in the oropharynx are found to have Candida esophagitis. As a result, it can be extremely difficult to differentiate viral and fungal esophagitis on clinical grounds.
The natural history of herpes esophagitis is uncertain. In various autopsy series, it has been shown that immunocompromised hosts with herpes esophagitis may develop herpetic pneumonitis or even a disseminated herpetic infection. However, most patients with herpes esophagitis recover spontaneously. These individuals are usually treated effectively with analgesics and, if necessary, antiviral agents such as acyclovir.
Otherwise healthy patients with herpes esophagitis have a characteristic clinical presentation. They typically are young men with a history of recent exposure to sexual partners with herpetic lesions on the lips or buccal mucosa. Most of these patients have a 3- to 10-day influenza-like prodrome characterized by fever, sore throat, upper respiratory tract infection, and myalgias. This prodrome is followed by acute onset of odynophagia, which prompts the patient to seek medical attention. Despite the dramatic presentation, these patients almost always have an acute, self-limited illness, with resolution of symptoms in less than 2 weeks.
Endoscopic Findings
Herpes esophagitis is initially manifested on endoscopy by esophageal vesicles that subsequently rupture to form discrete, punched-out ulcers. With further progression, the ulcers may become covered by a fibrinous exudate. Thus, early herpes esophagitis has a characteristic endoscopic appearance, whereas advanced herpes esophagitis may be indistinguishable from candidiasis. Whatever the stage of infection, the histologic or cytologic findings on endoscopic biopsy specimens or brushings are relatively specific for the herpesvirus group. The classic finding of Cowdry type A intranuclear inclusions in intact epithelial cells adjacent to ulcers is virtually pathognomonic of herpes. The diagnosis of herpes esophagitis can also be confirmed by positive viral cultures from the esophagus or by direct immunofluorescent staining for the herpes simplex antigen.
Radiographic Findings
Herpes esophagitis is usually manifested on double-contrast esophagograms by multiple, small (<1 cm), superficial ulcers in the upper or midesophagus, without plaque formation. These ulcers are visible on double-contrast images in more than 50% of patients. The ulcers may have a punctate, linear, ringlike, or stellate configuration and are often surrounded by radiolucent mounds of edema ( Fig. 20-12 ). Although ulceration may occasionally be seen in advanced Candida esophagitis, the ulcers in these patients almost always occur on a background of diffuse plaque formation. Thus, in the appropriate clinical setting, the presence of multiple small, discrete ulcers in the upper or midesophagus should be highly suggestive of herpes esophagitis. Nevertheless, endoscopy may be required for a definitive diagnosis if the radiographic findings are equivocal or if appropriate treatment with antiviral agents fails to produce an adequate clinical response.