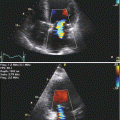
Fig. 7.1
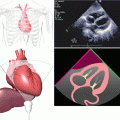
Apical four-chamber transthoracic echocardiography (TTE) showing large vegetation attached to the posterior leaflet of the mitral valve (arrows)
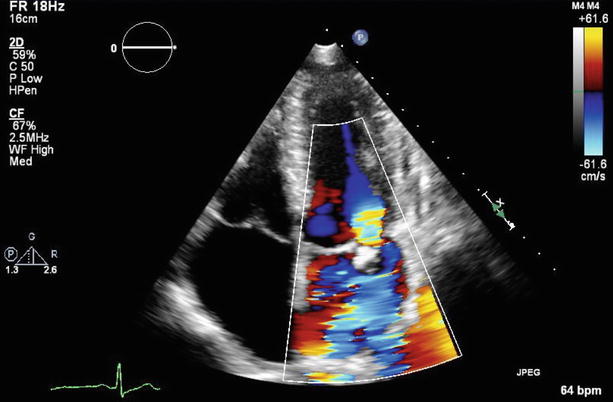
Fig. 7.2
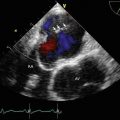
Color Doppler imaging of Fig. 7.1 demonstrated severe mitral regurgitation with an eccentric, anteriorly directed jet
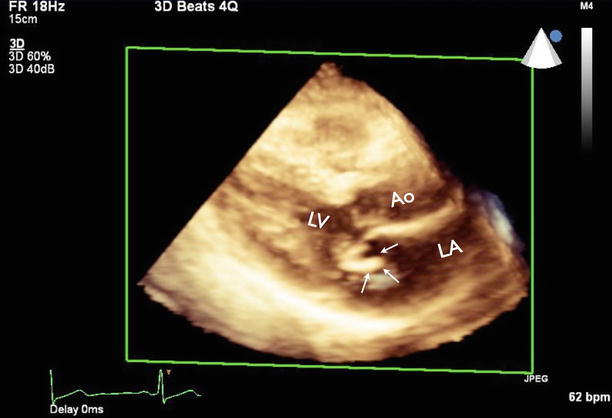
Fig. 7.3
Three-dimensional TTE showing vegetation at the posterior mitral leaflet (arrows). Ao aorta, LA left atrium, LV left ventricle
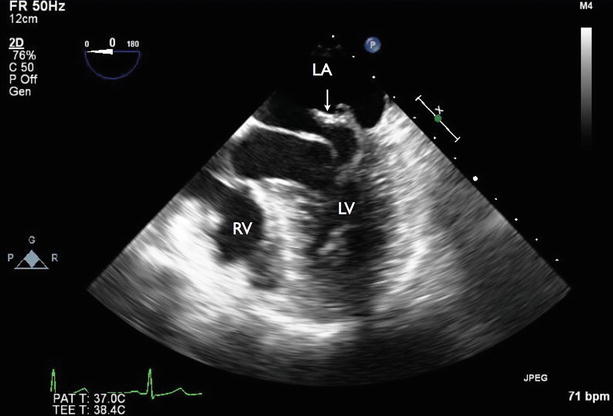
Fig. 7.4
Four-chamber transesophageal echocardiography (TEE) showing flail of the P2 segment of the posterior mitral leaflet (arrow). RV right ventricle
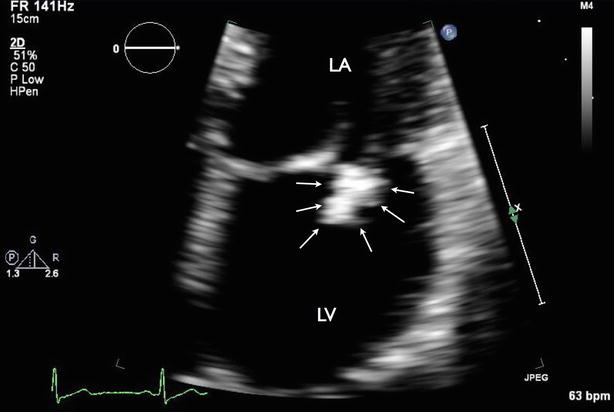
Fig. 7.5
Zoom view of mitral valve showing large vegetation at the posterior mitral leaflet (arrows)
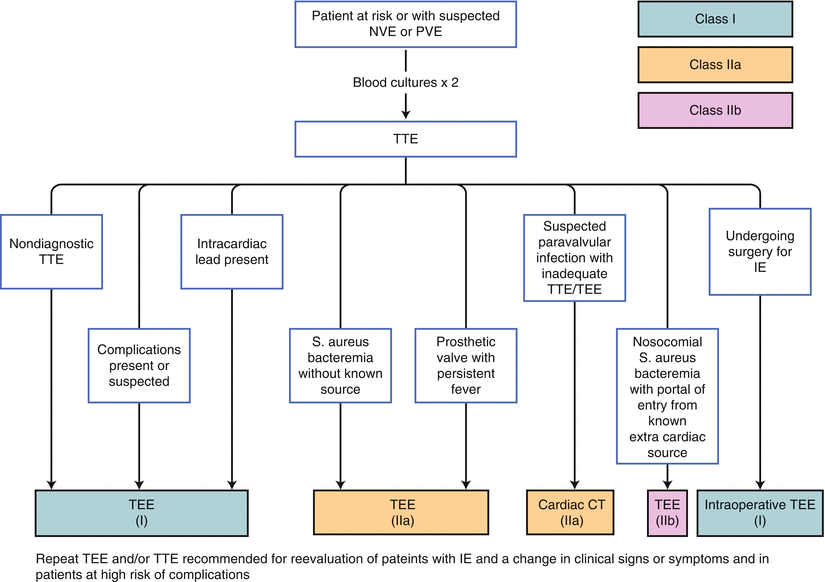
Fig. 7.6
Appropriate use of multimodality imaging in infective endocarditis according to 2014 ACC/AHA guidelines for management of valvular heart disease. IE infective endocarditis, NVE native valve endocarditis, PVE prosthetic valve endocarditis, S. aureus staphylococcus aureus (From Nishimura et al. [1])
Video 7.1 Apical four-chamber transthoracic echocardiography (TTE) showing large vegetation attached to the posterior leaflet of the mitral valve (AVI 8904 kb)
Video 7.2 Color Doppler imaging of Fig. 7.1 demonstrated severe mitral regurgitation with an eccentric, anteriorly directed jet (AVI 2018 kb)
Video 7.3 Three-dimensional TTE showing vegetation at the posterior mitral leaflet (AVI 2539 kb)
Video 7.4 Four-chamber transesophageal echocardiography (TEE) showing flail of the P2 segment of the posterior mitral leaflet (AVI 6317 kb)
Video 7.5 Zoom view of mitral valve showing large vegetation at the posterior mitral leaflet (AVI 17801 kb)
7.1.1 Learning Points
For the diagnosis of infective endocarditis (IE), TTE is considered the first-line cardiac imaging modality to demonstrate valvular vegetation, leaflet destruction, and complications of IE. TTE has a sensitivity between 50 and 90 % and a specificity greater than 90 % for detection of vegetations in native valve endocarditis (NVE). Transesophageal echocardiography (TEE) has a sensitivity ranging from 90 to 100 % for detection of NVE. TEE is recommended for all patients with known or suspected IE when TTE is nondiagnostic, when complications have developed or are clinically suspected, or when intracardiac device leads are present [1].
Mitral valve vegetation typically is located on the upstream side of the leaflet (i.e., the atrial side) but also can be attached to the chordae or the posterior wall of the left atrium. Echocardiographic features to define vegetation include a highly mobile mass that is typically irregularly shaped and low-reflectant, with rapid independent motion, prolapse into the left atrium during systole, and functional evidence of valve destruction.
7.2 Case 2. Native Valve Infective Endocarditis with Complications
A 60-year-old woman with a history of hypertension, intravenous drug abuse, and end-stage renal disease on hemodialysis was admitted to the hospital because of progressive dyspnea. Laboratory tests showed leukocytosis, and a chest x-ray showed diffuse bilateral pulmonary edema. Two sets of blood cultures grew methicillin-resistant Staphylococcus aureus (MRSA). TTE was performed and showed 60 % left ventricular ejection fraction with new, moderate mitral regurgitation and probable vegetation on the anterior mitral leaflet. She was treated with intravenous vancomycin for mitral valve IE.
Two days later, she developed acute-onset chest pain; electrocardiography showed diffuse ST segment elevations in the anterior, inferior, and lateral leads with elevated cardiac troponin T (TnT). She was transferred to our institute. Her cardiac catheterization revealed distal left anterior descending (LAD) embolism likely due to IE. She was supported on an intra-aortic balloon pump. She later developed first-degree AV block and disseminated intravascular coagulopathy (DIC), which required several vasopressors to maintain her hemodynamics. With her critically ill status and comorbidities, she was deemed too high-risk for open heart surgery and died a few days later (Figs. 7.7, 7.8, 7.9, 7.10, 7.11, 7.12, 7.13, and 7.14).
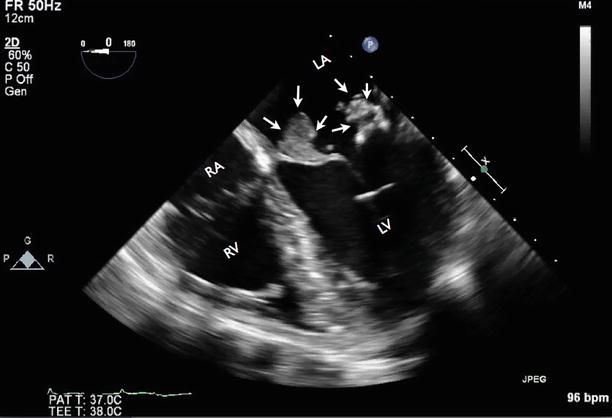
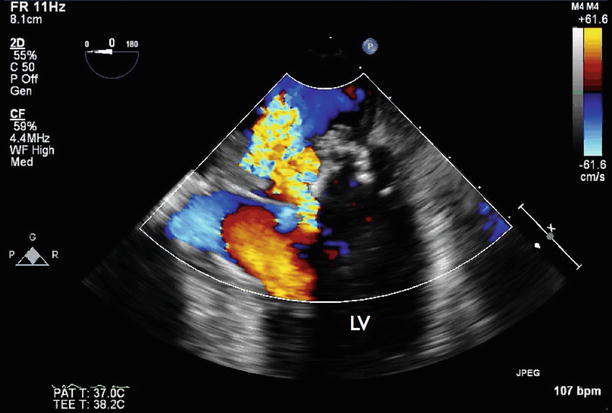
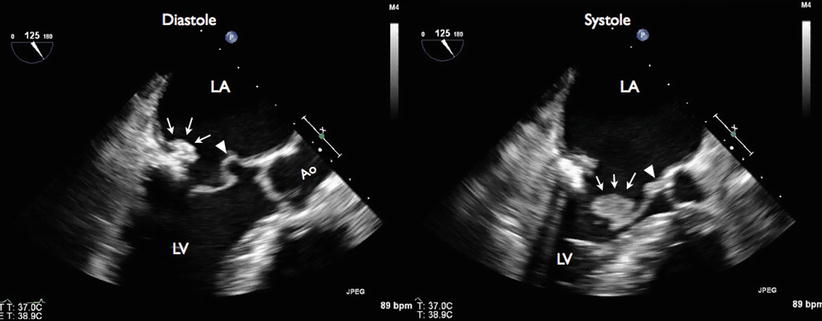
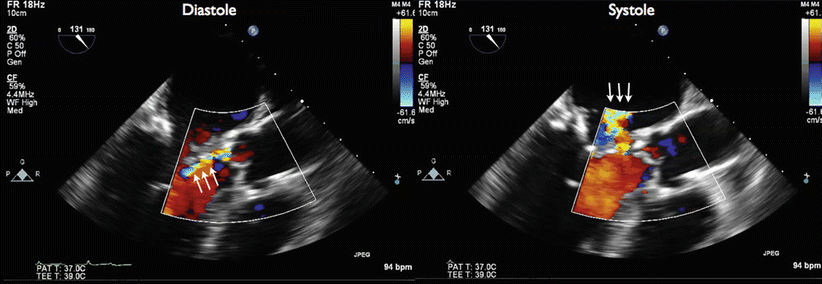
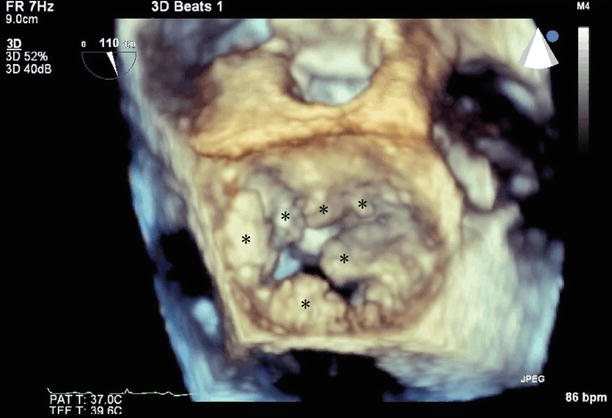
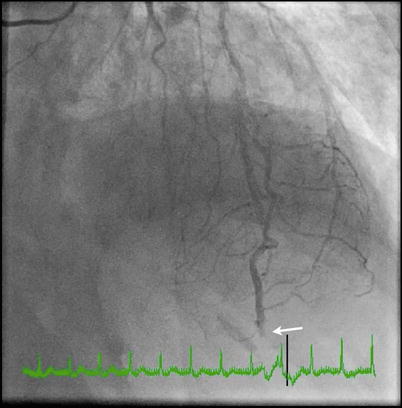
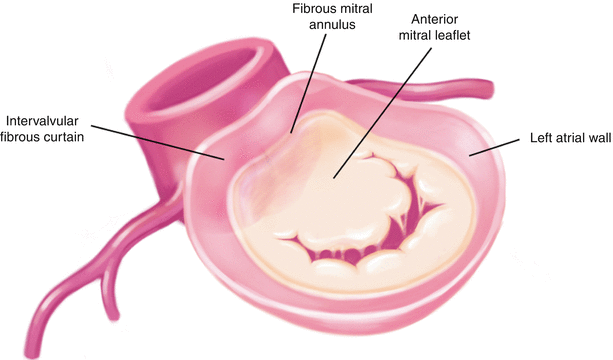
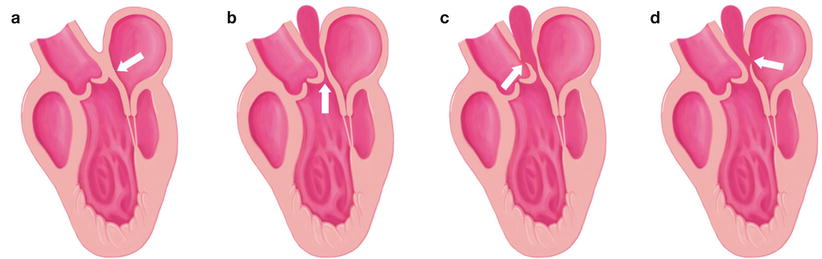

Fig. 7.7
Apical four-chamber TEE showing a large vegetation attached to the anterior and posterior leaflet of the mitral valve (arrows). RA right atrium

Fig. 7.8
Apical four-chamber TEE with color Doppler flow imaging showing severe mitral regurgitation

Fig. 7.9
Long-axis TEE demonstrated posterior mitral leaflet vegetation and mitral-aortic intervalvular fibrosa abscess and aneurysm. During diastole (left panel), vegetation was seen attached to the P2 segment of the posterior mitral leaflet (arrows), with outpouching of the proximal A2 segment of anterior mitral leaflet at the aortomitral curtain area, a finding consistent with mitral-aortic intervalvular fibrosa pseudoaneurysm (arrowhead). During systole (right panel), vegetation was seen attached to the P2 segment of the posterior mitral leaflets (arrows). There is a thickening of the aortomitral curtain area, with an area of hyperechodensity consistent with aortic abscess (arrowhead)

Fig. 7.10
Long-axis TEE with color Doppler flow imaging showing mild aortic insufficiency and perforation of the anterior mitral leaflet. During diastole (left panel), a mild aortic insufficiency (AI) jet was seen (arrows). The AI jet was eccentric and anteriorly directed. During systole (right panel), there was mitral regurgitation caused by leaflet perforation from a “kissing lesion.”

Fig. 7.11
Three-dimensional (3D) TEE of the mitral valve (LA view) showing multiple vegetations (asterisks)

Fig. 7.12
Coronary angiogram showing total occlusion of the distal left anterior descending artery (LAD) (arrow). Given the angiographic features of acute cut-off appearance and the history of multiple vegetations, the finding is most likely consistent with coronary embolism from IE

Fig. 7.13
Schematic diagram showing the close anatomical relationship of the mitral and aortic valves and the location of the intervalvular fibrous curtain

Fig. 7.14
The normal aortomitral curtain and several types of aortomitral curtain pseudoaneurysms. (a) Normal aortomitral curtain (arrow). (b) Aortomitral curtain pseudoaneurysm (arrow). (c) Aortomitral curtain pseudoaneurysm with fistula to the aorta (arrow). (d) Aortomitral curtain pseudoaneurysm with fistula to the left atrium
Video 7.6 Apical four-chamber TEE showing a large vegetation attached to the anterior and posterior leaflet of the mitral valve (AVI 4594 kb)
Video 7.7 Apical four-chamber TEE with color Doppler flow imaging showing severe mitral regurgitation (AVI 837 kb)
Video 7.8 Long-axis TEE demonstrated posterior mitral leaflet vegetation and mitral-aortic intervalvular fibrosa abscess and aneurysm (AVI 4764 kb)
Video 7.9 Long-axis TEE with color Doppler flow imaging showing mild aortic insufficiency and perforation of the anterior mitral leaflet. During diastole, a mild aortic insufficiency (AI) jet was seen, which was eccentric and anteriorly directed. During systole, there was mitral regurgitation caused by leaflet perforation from a “kissing lesion.” (AVI 1713 kb)
Video 7.10 Three-dimensional (3D) TEE of the mitral valve (LA view) showing multiple vegetations (AVI 1022 kb)
Video 7.11 Coronary angiogram showing total occlusion of the distal left anterior descending artery. Given the angiographic features of acute cut-off appearance and the history of multiple vegetations, the finding is most likely consistent with coronary embolism from IE (AVI 5517 kb)
7.2.1 Learning Points
This case demonstrated two serious complications of mitral valve infective endocarditis: coronary embolism and pseudoaneurysm of the aortomitral curtain. Septic embolism leading to an acute myocardial infarction is rare, and balloon angioplasty with emergent cardiac surgery has been proposed as a treatment option. It can be argued that balloon dilation and stent implantation should be avoided, to prevent distal embolization and entrapment of septic embolism material in situ, which may affect vascular integrity in the long run [5, 6].
Because of the close anatomical relationship between the mitral and aortic valves, the infection can start from either valve and extend toward the other. There are two main mechanisms of extension of the infection from the aortic valve to the mitral valve: (1) aortic annular abscess that extends to the aortomitral curtain or intervalvular fibrous curtain and anterior mitral leaflet (Fig. 7.13), and (2) the aortic regurgitation caused by IE of the aortic valve, causing a “kissing lesion” on the secondarily infected anterior mitral leaflet. Various types of pseudoaneurysm of the aortomitral curtain are shown in Fig. 7.14. Endocarditis can also extend from the mitral to the aortic valve, by direct extension of a mitral annular abscess or as an isolated satellite lesion. The vegetation is usually small, typically located on the ventricular side of a leaflet, and does not result in aortic valve regurgitation. Comprehensive analysis of the aortic valve is mandatory in all patients with mitral valve endocarditis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree