Childhood interstitial lung disease (chILD) in children, teenagers, and young adults presents a challenge to the clinicians and radiologist, given its rarity, diverse imaging manifestations, and often nonspecific clinical examination findings. This article discusses the utility of available imaging techniques and associated characteristic imaging findings, and reviews the 2015 chILD classification scheme, with clinical examples highlighting the imaging features to help the radiologist aid in an efficient and accurate multidisciplinary diagnosis of chILD.
Key points
- •
Interstitial lung disease (ILD) in children, adolescents, and young adults is a heterogeneous group of disorders with diverse clinical and imaging manifestations.
- •
Although childhood ILD (chILD) pathologies in infants and young children do overlap with ILD in adults, major differences in pathology and clinical presentations exist.
- •
ILD in this population remains a challenging and multidisciplinary diagnosis, with no standardized approach to diagnosis and management.
- •
It is imperative to establish the immune status of the patient when evaluating suspected ILD in this population, as it affects the pathology that can manifest and prognosis.
Introduction
Interstitial lung disease (ILD) consists of a large and heterogeneous group of rare pulmonary disorders, characterized by abnormalities involving the alveoli and airway. However, as many of these pathologies involve beyond or do not involve the interstitium at all, ILD in children and infants (chILD) is often considered a syndrome of diffuse ILD. Underlying chILD pathologies are markedly different from adult ILD. For example, the adult ILD idiopathic pulmonary fibrosis, or the corresponding pathologic diagnosis of usual interstitial pneumonia, has not been convincingly reported in children or teenagers, and conversely, the chILD disorders neuroendocrine cell hyperplasia in infancy (NEHI) and pulmonary interstitial glycogenosis have not been reported in adults.
The widely accepted chILD classification was initially developed and published in 2007, through the multidisciplinary collaborative efforts of the chILD Research Co-operative of North America (chILDRN), based on review of lung biopsies of 187 infants from 11 pediatric institutions with diffuse lung disease. , Although this classification was widely accepted and incorporated into official American Thoracic Society (ATS) clinical practice guidelines in 2013, it only systematized patients younger than 2 years.
Subsequently in 2015, chILDRN published an expanded classification to include older children from 2 to 18 years of age. The updated classification was based on a retrospective review of lung biopsies in 191 patients between 2 and 18 years of age from 12 North American institutions with diffuse lung disease. This classification divides patients into immunocompromised and immunocompetent clinical status ( Table 1 ), and further classifies the immunocompetent patients into primary lung disease, lung disease related to systemic disease, and patients with sequelae or diagnoses of disorders of infancy (see Table 1 ). Immunocompromised patients had the highest reported mortality rate of 52.8%, whereas immunocompetent patients had markedly improved mortality rates, with reported mortality rates ranging from 7.1% to 20.0% in the subgroups.
| Category | Disease |
|---|---|
| Immunocompetent: | |
| Primary lung disease in immunocompetent host |
|
| Lung disease related to systemic disease |
|
| Sequelae and ongoing disorders of infancy |
|
| Immunocompromised: | |
| Immunocompromised host |
|
Patients with chILD most commonly present with cough, exercise intolerance, dyspnea, hypoxemia, crackles, and tachypnea, although rarely, patients can present with a normal examination. , Interestingly, Fan and colleagues reported that clinical symptoms are less common in the older population, in comparison with the previously reported prevalence in infants younger than 2 years, implying that older children may have more insidious symptoms and present later in disease, making the clinical diagnosis a challenge.
Given its rarity and diverse imaging manifestations, and in conjunction with an often nonspecific clinical examination, chILD in children and teenagers presents a challenge to the clinicians and radiologist. Therefore, this article discusses the utility of available imaging techniques and the associated common imaging findings, and reviews the 2015 chILD classification scheme with clinical examples highlighting the imaging features to help the general radiologist aid in an efficient and accurate multidisciplinary diagnosis of chILD.
Imaging techniques
Chest Radiography
Chest radiography is an excellent initial screening imaging modality for patients with suspicion of chILD, as it uses low radiation dose, is easily reproducible, and readily accessible. , Once a chILD diagnosis has been established, it also can be used to follow the course of disease. , The most commonly seen radiographic abnormality is hyperinflation, although chest radiographic findings remain nonspecific, and a normal chest radiograph does not exclude a diagnosis of chILD. , , Prior studies have reported an inferior degree of confidence and accuracy of chest radiography in the assessment of diffuse pediatric lung disease, with reported accuracies as low as 34%. Therefore, further characterization to improve diagnostic accuracy and confidence with computed tomography (CT) is typically necessary.
Computed Tomography
CT, usually without intravenous contrast and ideally performed as high-resolution CT (HRCT) technique, has become the standard imaging modality for evaluation of suspected chILD, as it allows confirmation of disease, superior characterization of the extent and distribution of disease, and identification of any unique imaging features. , In addition, as chILD can often be patchy, CT can recommend an ideal biopsy site and guide preoperative planning. ,
In more recent years with the CT technologic advances, volumetric high-resolution scanning of the entire chest can be performed in seconds. , Thus, older patients typically do not need to be sedated, and can be imaged during quiet respiration or following breath-hold maneuvers, , , as general anesthetic should be avoided because it is invasive, expensive, subject to procedural risks, and possibly associated with adverse neurocognitive effects. , , Another challenge in imaging children with general anesthesia is that the pathology can be obscured, as children are particularly prone to atelectasis due to high chest wall compliance and underdeveloped collateral ventilation system (pores of Kohn and channels of Lambert). , Additional challenges in the younger child include lower lung volumes, associated with poor inspiration, small patient size, and rapid respiratory motion.
CT imaging manifestations of chILD remains diverse, but typically includes nodules, ground-glass opacification, consolidation, air-trapping, cysts, interlobular and intralobular septal thickening, linear and reticular markings, and architectural distortion and traction bronchiectasis in fibrotic disease. , , These CT imaging descriptors have specific terminology and definitions as per the Nomenclature Committee of Fleischner Society.
Pulmonary nodule
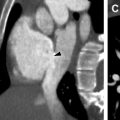
Pulmonary nodules can be classified according to their distribution as either centrilobular, miliary, perilymphatic, and random. Centrilobular nodules are found centrally within the secondary pulmonary lobule, and can be seen in infectious and inflammatory entities involving the small airways, such as infectious bronchiolitis or hypersensitivity pneumonitis. In contrast, perilymphatic nodules are found along the interlobular septa, fissures, and bronchovascular bundles, and can be seen in sarcoidosis ( Fig. 1 ). Miliary and random nodular patterns are not commonly seen in chILD.

Ground-glass opacification
Ground-glass opacification is visualized as hazy air space opacification with preservation of the bronchovascular structures, whereas consolidation represents solid air space opacification , ; however, both imaging characteristics are nonspecific. Ground-glass opacification can be seen in underinflated lungs, especially in the lung bases when imaged in expiration or with shallow inspiration. When ground-glass opacification is seen with superimposed interlobular and intralobular septal thickening, it is described as a “crazy-paving” pattern ( Fig. 2 ), and can be associated with a diverse range of entities, including pulmonary alveolar proteinosis (PAP), pulmonary hemorrhage syndromes, lipoid pneumonia, diffuse alveolar disease, organizing pneumonia, and pneumocystis pneumonia. ,

Air-trapping
Air-trapping appears as hypodense regions with less than normal increase in attenuation and lack of normal volume reduction with expiration ( Fig. 3 ). It is not uncommon to see a few subsegmental (lobular) hypodense foci in the lungs of healthy children, especially in the posterior juxta-pleural region, and should not be confused with pathology.

Cyst
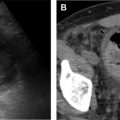
Cysts appear as round hypodense, well-defined structures that usually contain air, but can contain fluid or solid material. Cysts can be seen in multiple chILD entities, including pulmonary Langerhans cell histiocytosis (LCH) ( Fig. 4 ) and disorders of surfactant metabolism (see Fig. 19 ).

Pulmonary fibrosis
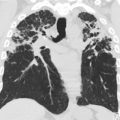
Pulmonary fibrosis associated with chILD and ILD can develop, and manifest on CT as thicker-walled stacked cysts known as honeycombing, in addition to traction bronchiectasis and architectural distortion ( Fig. 5 ). ,

Magnetic Resonance Imaging
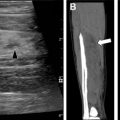
MR imaging is a superb imaging modality for assessment of the mediastinum and chest wall, and offers the absence of ionizing radiation. However, it remains limited in the imaging of chILD. This is mainly due to a combination of low proton content in the lungs, inevitable respiratory motion artifact as the images are obtained over a period of free breathing, and inferior spatial resolution relative to CT. Sodhi and colleagues previously investigated the utility of MR imaging in the evaluation of chILD in comparison with HRCT, and found that 3T MR imaging was able to detect consolidation ( Fig. 6 ), parenchymal bands, and fissural thickening, but remained limited in evaluation of septal thickening, ground-glass opacity, nodules ( Fig. 7 ), and cysts, which often are diagnostic and differentiating features of chILD. Therefore, at the current time, MR imaging remains limited in the clinical application of chILD, particularly for initial evaluation.


Challenging aspects of interstitial lung disease in adolescents and young adults for general radiologists in 2019
chILD remains a complex diagnosis, and the reported accuracy and diagnostic confidence of a correct diagnosis using HRCT in children is lower than in adults, mostly thought to reflect the greater diversity of lung diseases in the pediatric population. In addition, it is important to recognize that the ability to determine the correct diagnosis is dependent on the type of disease, the quality of the study, and the expertise of the interpreter. Even pediatric thoracic radiologists may only achieve a correct first-choice diagnosis of chILD in fewer than 50% of cases, although prior diagnostic performance studies are confounded by inconsistent results and imprecise and outdated histopathologic classification schemes. Therefore, it is imperative to use a multidisciplinary approach among the clinician, radiologist, and pathologist in the assessment of patients with suspected chILD to optimize diagnosis and management.
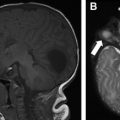
Although challenging due to the diverse pathologies and variable imaging manifestations, the essential role as a radiologist is to identify any unique features to suggest a favored or narrowed differential diagnosis. This can direct the next appropriate serologic assay, bronchoalveolar lavage (BAL), or genetic test, and potentially obviate the need for a lung biopsy, such as in the setting of NEHI or bronchiolitis obliterans in the appropriate clinical setting. , , , It can be highly beneficial when extrapulmonary imaging manifestations, such as thymic enlargement and calcification in LCH, esophageal dysfunction in systemic sclerosis or aspiration, and pectus excavatum in chronic surfactant dysfunction related to ABCA3 gene mutation are present and help suggest the diagnosis.
The other challenge lies in the substantial variation and absence of clear guidelines for monitoring patients with chILD with diagnostic imaging. This is because the imaging findings on CT do not always correlate with pulmonary function testing (PFT), or predict response to treatment or outcome. For example, patients with asymptomatic connective tissue disease with normal PFT often have ILD on imaging. , , Conversely, it is not unusual for asymptomatic HRCT lung abnormalities to persist in LCH for many years after treatment. , In addition, there are no specific HRCT findings to predict which patients progress to clinically significant pulmonary fibrosis. , ,
Spectrum of interstitial disease
Primary Lung Disease in an Immunocompetent Host
Infectious/postinfectious: constrictive obliterative bronchiolitis
Postinfectious constrictive obliterative bronchiolitis (COB) is a chronic respiratory condition characterized by severe and fixed lower respiratory airway obstruction due to inflammatory tissue and fibrosis, and associated with sequelae of prior infection from various respiratory viruses, but particularly with adenovirus. ,
On chest radiographs, hyperinflation is the most common abnormality, with additional findings including atelectasis and bronchial thickening, but milder disease also can have a normal chest radiograph. , , HRCT demonstrates characteristic mosaic attenuation, due to vascular shunting in the hypoventilated areas and reduced perfusion due to constriction of vessels from tissue hypoxia. On expiratory images, air-trapping is present, reflecting small airways disease, and mild to marked bronchiectasis, reflecting larger airways disease ( Fig. 8 ). , Although definitive diagnosis remains by lung biopsy, a 3-part COB diagnostic scoring system with a diagnostic specificity of 100% has been proposed, potentially obviating the need for lung biopsy, with points given for a typical clinical history of postinfectious COB, history of prior adenovirus infection, and characteristic mosaic attenuation on HRCT. , ,

Environmental agents: hypersensitivity pneumonitis
Hypersensitivity pneumonitis (HP), also known as extrinsic allergic alveolitis, is a form of immune-mediated ILD that develops in response to repeated inhalation of finely dispersed organic antigens. Acute, subacute, and chronic forms have been described, with repeated exposures potentially leading to irreversible lung damage. HP remains uncommon in childhood, with nonspecific symptoms often resulting in a delay in diagnosis. Affected pediatric patients with acute HP can present with symptoms mimicking a flu-like illness, including high fever, chills, dry cough, dyspnea, and malaise, whereas children with chronic HP, the more commonly reported form of HP, present with progressive and insidious nonspecific symptoms of exercise intolerance, cough, weight loss, and fever. , Although it can manifest identically on imaging to the adult form, in contrast to adult HP, which can result from a wide variety of occupational and environmental exposures to microbes, animal and plant proteins, and chemicals, HP in children most commonly results from repeated exposures to an array of birds, and is associated with an overall excellent prognosis. Additional antigens in HP in children include mold spores and methotrexate. ,
On chest radiographs and HRCT, acute HP classically shows ground-glass opacities ( Fig. 9 ), which can resemble pulmonary edema or pneumonia. , In the subacute phase of HP, HRCT shows poorly defined centrilobular nodules, ground-glass opacities, and evidence of air-trapping. , There is relative sparing of the upper lung zones in both the acute and subacute phases. In chronic HP, subpleural reticular markings, architectural distortion, and honeycombing related to pulmonary fibrosis are seen. , Although clinical symptoms typically resolve within a few days of starting treatment and ceasing exposure to the inciting antigen, HRCT findings of acute and subacute HP may persist for several weeks. Also, HRCT findings of pulmonary fibrosis may persist and potentially progress, even despite removal of the offending antigen. , ,