Chapter 34 Interventional and therapeutic procedures
Angioplasty
Iliac angioplasty
The results of iliac angioplasty are generally very good, with a low complication rate.1,2 The procedure is safe and successful, and in many centres it is offered to patients who have intermittent claudication after 100 m walking or less. It may also be of great value as an adjunct to surgery.3 For example, if a lower limb bypass graft is to be undertaken, iliac angioplasty to a stenosis above the proposed site of the proximal anastomosis will improve the inflow of blood, making a successful bypass more likely and reducing the extent of the surgery required.
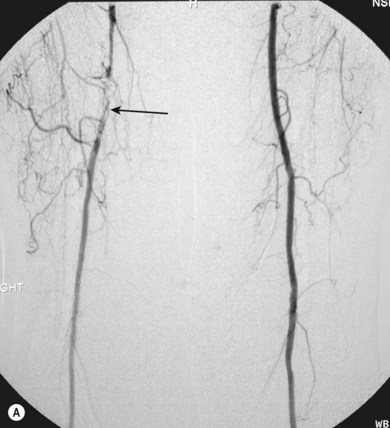
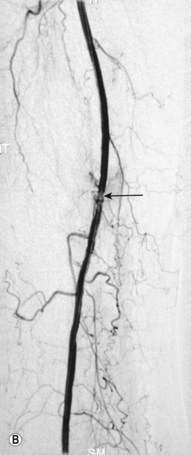
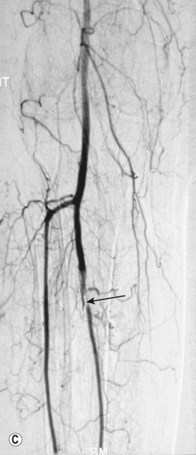
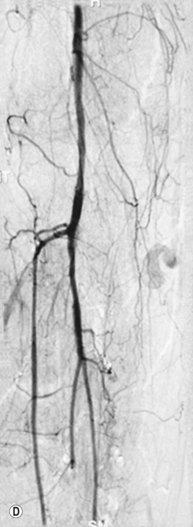
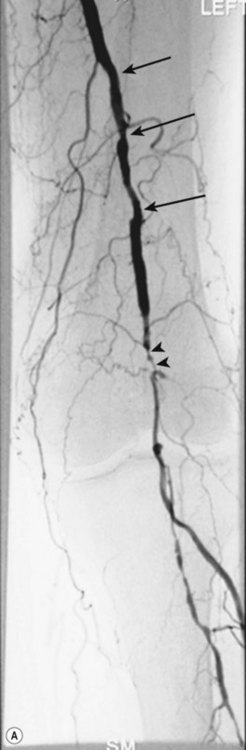
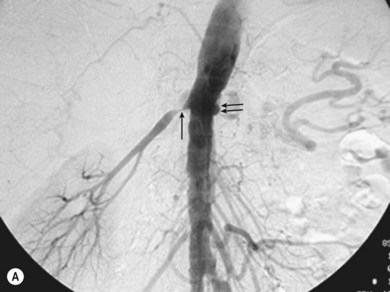
Superficial femoral artery (SFA) angioplasty (Fig. 34.1A–D)
This procedure is most commonly undertaken for the management of critical lower limb ischaemia or short-distance intermittent claudication. Such ischaemia is most likely to be caused by SFA occlusion, rather than a simple stenosis; thus to perform an angioplasty one must first cross the occlusion with a guide wire. This can be difficult, but the use of a hydrophilic guide wire will facilitate successful crossing in the vast majority of cases, with many operators electing to pass the guide wire subintimally. SFA angioplasty is less commonly performed for treatment of intermittent claudication, as generally the results are inferior to those of iliac angioplasty,4,5 and two randomised studies have shown that the results are no better over the long term than those observed after a supervised exercise programme.6,7
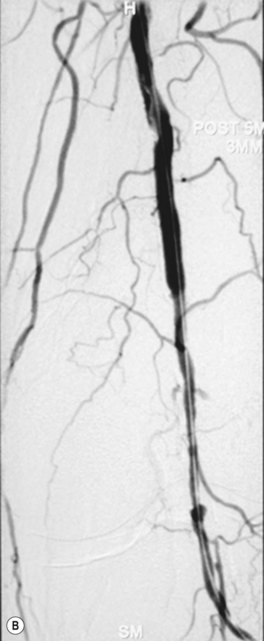
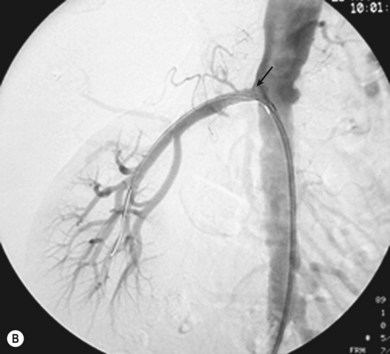
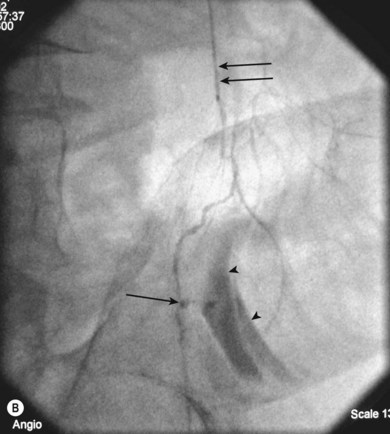
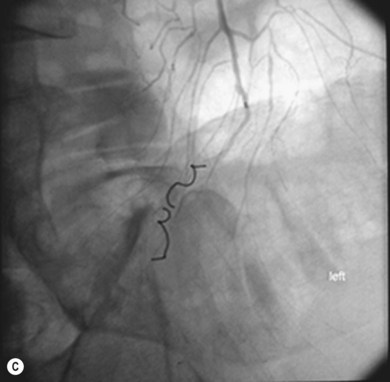
As with iliac angioplasty, SFA lesions can be approached either contralaterally or ipsilaterally. The contralateral approach is the same in technical terms as that used for the iliac vessels. However, the ipsilateral approach to the SFA is technically more difficult, as an antegrade puncture of the CFA is required. To perform an antegrade puncture, the femoral head is first identified under fluoroscopy and its position marked on the skin surface with a metal marker. Local anaesthetic is infiltrated into the skin over the femoral pulse as it is palpated at this level. A puncture needle is introduced first and a guide wire is then introduced along the SFA. It is possible that the guide wire may pass into the profunda femoris, and for this reason it is important to observe its progress under fluoroscopic control. If it proves difficult to enter the SFA, it may be necessary to screen over the needle tip while manipulating it into different positions to facilitate guide wire advancement. When doing this it is very easy for the operator to put their hands into the X-ray beam without realising. The radiographer can prevent or minimise this by centring only on the very tip of the needle, rather than its whole length, and using the collimators appropriately. Antegrade puncture is often used because the distance from the puncture site to the angioplasty site is short, avoiding the need to use very long guide wires. It also avoids any problems associated with catheter manipulation when dealing with tortuous iliac arteries or an acutely angled aortic bifurcation; in the event of a complication occurring, the subsequent management, e.g. aspiration embolectomy, is much more straightforward (Fig. 34.1C,D).
Popliteal artery and the tibial vessels
Lesions in these vessels will only be treated with angioplasty in the presence of critical lower limb ischaemia or short-distance claudication (Fig. 34.2A,B). The potential benefit of angioplasty at these sites in patients with uncomplicated intermittent claudication would be completely outweighed by the potential risk and the likely recurrence rate in the future.8 Technically there is very little difference between angioplasty performed here and elsewhere in the lower limb. Smaller diameter balloons are used, and many operators prefer to use finer guide wires.
Vascular stent insertion
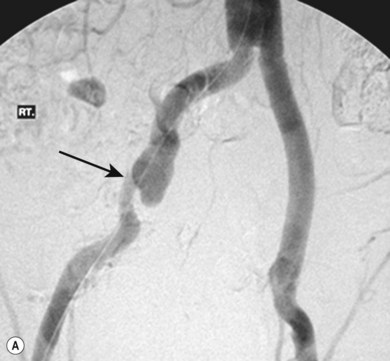
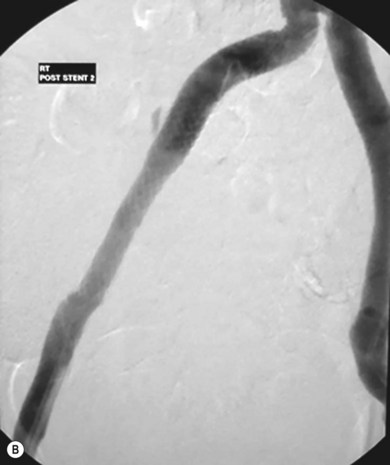
Stenting the iliac artery (Fig. 34.3A,B)
It has been shown that if iliac angioplasty is technically successful there is no advantage in terms of clinical outcome in adding a stent.9–11 However, in about 50% of cases the outcome from angioplasty is suboptimal, perhaps due, for example, to elastic recoil of the vessel wall or dissection causing flow limitation. Many professionals would add to this and include failure to reduce the intra-arterial pressure gradient across the lesion to less than 10 mmHg as an indication.
The exception to this is the treatment of iliac artery occlusions where, if angioplasty alone is used, there is an incidence of peripheral embolisation of up to 50%.9 For this reason, primary stenting is undertaken when treating iliac occlusions endovascularly. Thus, a self-expanding stent is first deployed across the occlusion and subsequently dilated using an angioplasty balloon.
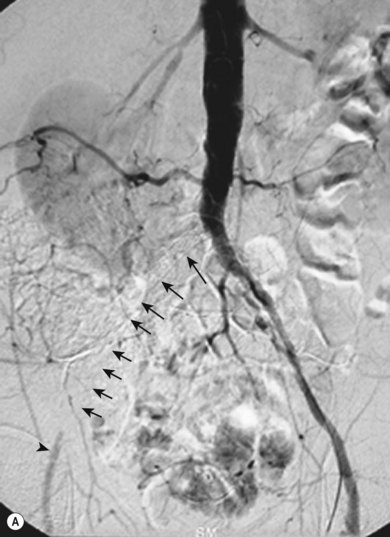
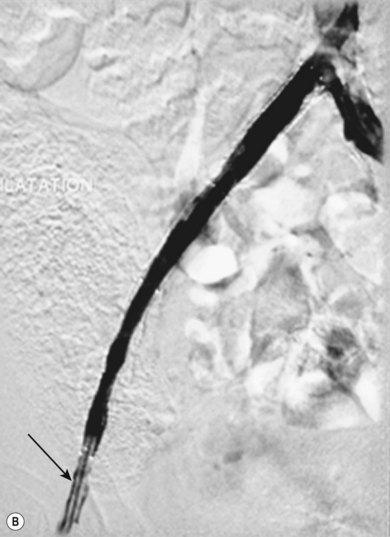
Stenting the renal artery (Fig. 34.4A,B)
Renal artery stenosis is generally caused by one of two pathologies, either fibromuscular hyperplasia or atheroma. Fibromuscular hyperplasia is an uncommon cause of uncontrollable hypertension and responds well to angioplasty alone. Atheromatous renal artery stenosis (ARAS), when it requires treatment, responds very poorly to angioplasty alone, and it has clearly been demonstrated that primary stenting is superior in both the short and the longer term.12 This happens because the vast majority of ARAS occurs at the origin of the vessel and is caused by aortic atheroma rather than true atheroma of the renal artery. Therefore, an expansile force applied to the stenosis causes shear stresses within the aortic plaque, rather than an expansile force within the renal artery lumen. Once the angioplasty balloon is removed the stenosis will frequently recur as the aortic plaque moves back into position.
Vascular stent grafts
As previously mentioned, stent grafts are used in the treatment of true or false aneurysms. The technology continues to evolve, and it is not possible to say at this point whether stent grafting will replace open surgery in the treatment of aneurysmal disease. However, there is growing evidence to support the use of stent grafts in the treatment of thoracic aortic aneurysms, where the risks of surgery are considerably greater than those of open surgery for abdominal aneurysms.13 Furthermore, there is some evidence to suggest that stent grafting may be of value in patients who would be at greater than average risk for abdominal surgery, for example if they have renal failure.14–16 More recently, randomised data have shown a reduction in 30-day mortality when stent grafts are used for abdominal aortic aneurysm repair, compared to open surgery.17,18
When used for aortic aneurysms, stent graft delivery systems are large and require surgical exposure of one or both common femoral arteries. Smaller aneurysms, such as in the iliac arteries, can be treated without surgical exposure of vessels (Fig. 34.5A,B). Therefore, aortic stent graft procedures are frequently performed in the operating theatre with a mobile image intensifier. A better alternative, which is becoming increasingly available, is to use an angiographic suite that has been constructed to operating theatre standards. This provides a sufficiently sterile environment with a high standard of imaging.
Embolisation
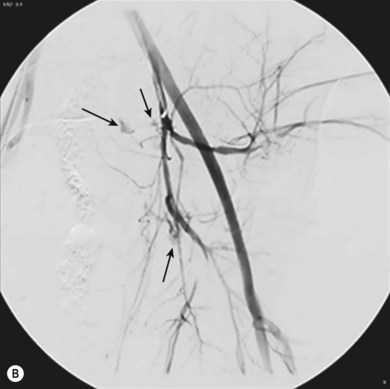
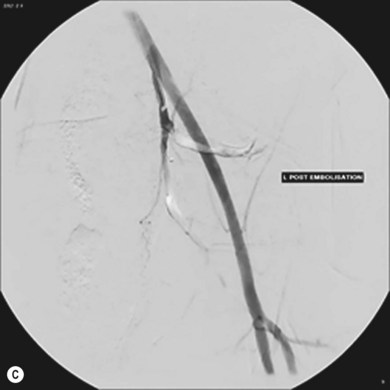
Commonly used embolisation agents include gelatin sponge (Fig. 34.6A–C) (for temporary embolisation), polyvinyl alcohol particles and coils (for permanent vessel occlusion). The full range of embolic materials available for clinical use is vast, complex, and includes materials that would require a whole chapter to describe and explain in detail. Embolisation procedures are often complex and time-consuming, and may require the use of superselective coaxial catheter systems; multiple magnified views of the area are needed.
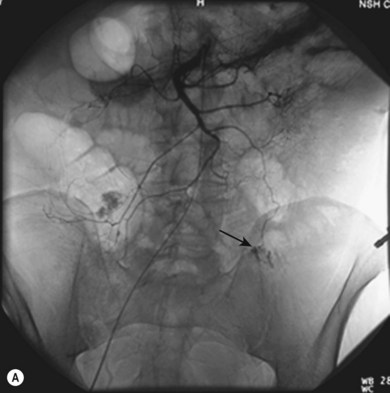
Other procedures are more complex, such as embolisation for gastrointestinal bleeding (Fig. 34.7A–C), and require a more flexible approach to determine the precise anatomy and demonstrate the bleeding point accurately, followed by therapy. Highly complex situations, such as therapy for arteriovenous malformations, may be better referred to centres with a specialist interest in this area.
Venous interventions
Tunnelled central venous lines
The best method of guiding the vessel puncture is US, which has the clear benefit of avoiding the use of ionising radiation and is recommended by NICE for jugular vein puncture.19 When performing subclavian vein puncture it is possible to opacify the target vein with contrast, and guide the puncture in this way. Fluoroscopy is used to identify the catheter tip when positioning it in the superior vena cava (SVC). The first choice of vein for puncture is the right internal jugular. This vein follows an almost straight course into the right brachiocephalic vein and subsequently the SVC, meaning that there is little potential for kinking of the introducer sheath during insertion. Use of the left internal jugular and subclavian veins is usually straightforward, whereas the use of the right subclavian vein can be difficult, as kinking of the introducer sheath can be a significant problem here.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree