An ideal imaging guidance system for cardiovascular, catheter-based interventional procedures would offer real-time, high-resolution, three-dimensional (3D) imaging of important anatomic tissues and chambers, irrespective of respiratory, cardiac, or patient motion alongside excellent visualization of catheters, guidewires, and other interventional devices. Such tools would quickly enable novel, minimally invasive alternatives to open surgical procedures.
X-ray fluoroscopy guides most contemporary catheter-based procedures. However, fluoroscopy has important limitations ( Table 47.1 ). Iodinated radiocontrast, which provides the ability to outline chamber and vascular lumens, is injected only periodically. Tissue detail is minimal. Additionally, fluoroscopy provides only two-dimensional (2D) “projection” imaging, with limited depth perception. Iodinated radiocontrast is nephrotoxic in susceptible individuals. Ionizing radiation increases lifetime cancer risk, particularly in children. Finally, operators and personnel risk disabling orthopedic injuries from the weight of protective lead apparel.
| Imaging Modality | Advantages | Disadvantages |
|---|---|---|
| iCMR |
|
|
| X-ray fluoroscopy |
|
|
| Ultrasound |
|
|
Ultrasound is often used in combination with x-ray fluoroscopy procedures. For example, transesophageal echocardiography (TEE) provides adjunctive imaging during atrial septal defect closure or transcatheter aortic valve replacement, by assisting with device sizing, positioning, and postdeployment assessment. However, transthoracic echocardiography (TTE) offers limited acoustic windows, and suffers “shadowing” artifacts caused by devices.
Cardiovascular magnetic resonance (CMR) more closely approaches the idealized imaging guidance system described earlier. CMR offers superior tissue imaging, in any arbitrary orientation, without ionizing radiation or nephrotoxic contrast. Rapid imaging techniques and modified CMR visible devices now permit real-time CMR guided catheter applications. The ability to visualize both tissue and interventional devices may enable catheter-based procedures in the future that currently require open surgical exposure. In the meantime, CMR right heart catheterization provides superior hemodynamic characterization combining high fidelity flow, pressure, and function measurements.
Interventional Cardiovascular Magnetic Resonance Laboratory Configuration
Early real-time magnetic resonance imaging (MRI) systems employed low field strength magnets (0.2–0.5 T), open or closed bore configurations, and sometimes embedded x-ray fluoroscopy systems. Major limitations included relatively low signal-to-noise ratio (SNR), inhomogeneous magnetic field interfering with rapid imaging, and limited patient access when employing long, closed bores. Newer, short-bore, 1.5 T systems with high performance gradients provide excellent field homogeneity and higher SNR. Newer scanners also include large arrayed radiofrequency (RF) receivers (≥32), intended for parallel imaging techniques to improve imaging speed. These additional receiver channels can also be used to attach “active” catheter devices to improve their visibility. By contrast, higher field 3 T systems disproportionately increase heating but do not notably enhance image quality using workhorse (balanced steady state free precession [bSSFP]) pulse sequences, and are therefore difficult to justify for interventional CMR (iCMR) applications.
In addition, commercial vendors now provide interactive scan user interfaces that increasingly resemble echocardiography. These interfaces drive sophisticated image reconstruction hardware and software that permit images to be acquired with ease and displayed to operators with minimal delay in multiple scan planes.
Combined x-ray and CMR catheterization laboratories, so-called XMR labs, now enable rapid and convenient CMR catheterization procedures, especially in pediatrics and heart failure ( Fig. 47.1 ). In these laboratories, conventional x-ray fluoroscopy guidance is immediately available to perform interventions using CMR fusion imaging or for situations requiring emergency bailout. XMR laboratories are now commercially available. For example, the National Heart Lung and Blood Institute (NHLBI) laboratory at Children’s National Medical Center in Washington, DC, combines a biplane x-ray fluoroscopy system with a 1.5 T cardiac scanner ( Fig. 47.1 ). These systems are separated by RF-shielded doors and can be used independently or together for combined procedures. An automated transport table moves patients seamlessly between the two modalities. Alternative designs include single-room layouts, in which both x-ray and CMR systems are located inside the RF shield. Wave guides and penetration panels are strategically positioned in the room so that communication and patient monitoring hardware can be installed without disrupting the RF shield.

Communication, Monitoring and Image Display
Rapid CMR requires rapid gradient switching that causes substantial acoustic noise. This noise prevents verbal communications between operators, the patient, and staff. Wireless noise-cancelling headsets are used at NHLBI ( Fig. 47.2 ). This versatile system allows staff and patients to speak simultaneously on open microphones and allows separate and parallel conversations with patients and among staff.

Gradients, RF interference, and magnetohydrodynamic effects severely distort the electrocardiogram (ECG). Electronic filters enable heart rate monitoring; however, ECG waveform morphology is not readily interpretable for ST-segment monitoring of myocardial ischemia or injury. Multichannel invasive pressure waveform monitoring, temperature monitoring, and oxygen saturation monitoring can use commercial systems, without modification from x-ray catheterization laboratories. Several manufacturers are developing dedicated hardware that will interface with existing catheterization laboratory high-fidelity hemodynamic recording systems. In the meantime, open source kits for local assembly of suitable hemodynamics interfaces are available ( http://nhlbi-mr.github.io/PRiME ).
Interventional catheterization suites require continuous operator monitoring of multiple information streams, including hemodynamics, imaging control, and interventional images. This is similarly true during iCMR procedures, especially using active devices. MRI-conditional liquid crystal display (LCD) video displays are available from most scanner manufacturers. We prefer two banks of less expensive LCD video projectors that project onto a fabric screen, which depict hemodynamics, scanner interface, and 3D-rendered images separately, one at each end of the CMR system ( Fig. 47.2 ). The projectors are housed in RF-shielded boxes and video signals are passed into the room via fiber optic cables through the waveguide.
Interventional Cardiovascular Magnetic Resonance Real-Time Scanner Interface
The iCMR scanner user interface requires several key features not typical for diagnostic CMR. Commercial or investigational graphic user interface based scanning host computer systems are available from most systems vendors (Interactive Front End, Siemens ; MR Echo, General Electric; eXTernal Control, Philips Healthcare) and as standalone commercial (RT Hawk, Heart Vista) or open-source solutions (Vurtigo, Sunnybrook Health Sciences Center, http://www.vurtigo.ca ).
One of the most useful features is the colorized display of individual receiver channels that are attached to “active” catheter receiver coil devices. This makes catheter devices readily apparent and has proven useful in vivo. Another is interactive “projection-mode” imaging wherein slice select is disabled and catheter devices are displayed as the operator is used to seeing them under conventional x-ray fluoroscopy. For tracking balloon catheters filled with dilute gadolinium (Gd) contrast during CMR catheterization, we find it helpful to interactively adjust slice thickness and saturation magnetization preparation, to help find the catheter tip should it move outside of the selected plane, and to interactively adjust temporal resolution for catheterization steps requiring fine motor control. Operationally, we find it useful to display multiple slices during interventional procedures, both separately and combined in a 3D-rendered image ( Fig. 47.3 ). Other laboratories are experimenting with voice-control, automatic adjustment of slice location to correspond to catheter position, and automatic adaptation of imaging parameters to speed of catheter manipulation.

Safety Considerations
Patient care staff must undergo formal comprehensive training in CMR safety. For example, the hospital emergency medical response team is unlikely to be familiar with CMR operations and may unwittingly jeopardize themselves, their colleagues, or the patient by rushing into the room with a steel oxygen tank. Rapid patient evacuation from the CMR catheterization laboratory needs to be practiced repeatedly in mock drills. Ferromagnetic objects that cannot be removed from the room must be attached to the wall during CMR guided procedures. It is good practice for all devices on wheels in the x-ray section of the iCMR suite (e.g., anesthetic machine, intraaortic balloon pump, or defibrillator) to be tethered to the wall or floor. Oxygen, inhalational anesthetic, and vacuum for suction can be fed through RF-protective wall ports, eliminating in-room cylinders. At NHLBI, we drill emergency evacuation from the CMR room on a quarterly basis. Swift evacuation relies on clear delineation of roles for all staff. In an emergency, we can start chest compressions within 10 seconds and move the patient back into the x-ray room for defibrillation in under 1 minute. In the near future, CMR-conditional external defibrillators should be commercially available so that arrhythmias can be managed without evacuating the patient from the scanner.
RF energy from CMR transmit coils will concentrate on long conductive devices causing local heating, potentially leading to burns. Box 47.1 lists factors associated with heating, especially long conductive devices and cables. This complex problem is described later under “active” catheter devices. Interventionists must also take care that connections to intravascular coils as well as surface coils do not inadvertently form loops, which can cause patient burns.
Interventional Device Factors
- •
Length of conductive elements
- •
Geometric shape
- •
Orientation in the magnet
- •
Distance from the radiofrequency transmitter
- •
Physical proximity to tissues
- •
Insulation
Patient Factors
- •
Body mass and surface area
- •
Convective cooling of intravascular devices by local blood flow
- •
General body temperature
- •
Implanted conductive devices
- •
Tissue thermosensitivity
Magnetic Resonance Imaging Scanner Factors
- •
Field strength
- •
Pulse sequence
- •
Flip angle
- •
Scanning duty cycle
- •
Position relative to bore isocenter (closer is better)
Real-Time Imaging
Rapid CMR is required for invasive procedures, for catheter visualization, and for imaging of anatomic structures. Efficient image data sampling methods, parallel imaging, and coherent steady state techniques have enabled real-time CMR without significant degradation in image quality in the field of interest. Frame rates as high as 10 to 15 images per second are now possible using multichannel (≥32) coils that use parallel imaging techniques to achieve acceleration factors of three or more. In addition, interactive, real-time color flow imaging may supplement anatomic detail with critical physiologic detail, such as leaks or gradients, during therapeutic procedures and interventions. Slice orientation can automatically follow the tip of catheter devices as they move, so-called adaptive imaging, so that catheter features are kept in view during manipulation. These methods also can be applied automatically to alter scanning parameters such as field-of-view and temporal resolution.
Interventional Cardiovascular Magnetic Resonance Catheter Devices
Interventional devices must be conspicuous and clearly distinguishable from tissue to conduct therapeutic procedures. Conventional x-ray devices are generally unsuitable, because most incorporate steel braids to increase x-ray attenuation for visibility and to enhance catheter performance characteristics such as steerability, pushability, and trackability. The steel causes severe blooming or susceptibility artifacts that destroy much of the CMR image ( Fig. 47.4 ). Removing these ferrous components usually renders the catheter devices virtually invisible under CMR, and usually renders them floppy and mechanically unsuitable as catheters.

iCMR catheter devices are generally classified as passive or active. Passive devices have elements that cause discrete susceptibility imaging artifacts that darken images, or T1 shortening elements that brighten images. Alternatively, active devices have built-in micro-coils and electrical circuitry that allow the device to act as a RF receiver and/or transmitter.
Passive Devices
Stents and guidewires made from copper, dysprosium, cobalt–chromium alloy, nitinol, titanium, and platinum suffer less severe susceptibility artifacts compared with iron-alloys. They can be coated to improve biocompatibility. Carbon dioxide creates a dark CMR signal by excluding proton spins; carbon dioxide gas has been injected in humans for selective CMR angiography and has been used to fill balloon catheters for diagnostic CMR-guided catheter tracking in patients. Unfortunately, volume averaging makes it difficult to distinguish passive devices from neighboring anatomic features. Guidewires have been made out of nonmetallic polymers, and clinical cardiovascular interventions have been conducted using polymer guidewires. Polymer guidewires lack the trackability, torquability, and tactile responsiveness of metallic guidewires; a new segmented nitinol design overcomes these limitations.
Devices that appear bright are less vulnerable to volume averaging effects and are more easily visualized against anatomic background. Dilute Gd chelates such as gadopentate dimeglumine (Gd-DTPA) have been used to fill and coat catheters and balloons, offering bright device imaging. Ultimately, we have found this passive approach to be inferior in vivo compared with active catheter device visualization ( Fig. 47.5 ). Investigational, off-resonance, chemical-selective visualization using F-19 or C-13 and other hyperpolarized agents have been proposed for catheter tracking. Off-resonance effects have been exploited to track metallic catheter and guidewire components. Clinical diagnostic catheterization for hemodynamic studies can be conducted using dilute Gd-filled balloon catheters, iron-contrast filled, or CO 2 -filled catheters as an alternative.

Active Devices
Highly sensitive, ultrasmall receiver coils can be incorporated into devices to locate (catheter-tracking ) or to visualize them, or both. A minimum of two coils at the tip of a catheter is required to enable the computer to calculate the position of the catheter in 3D space and depict it on an image. This approach is particularly attractive to procedures that require high spatial location accuracy, for example electrophysiology mapping and ablation. Tracking also permits the user interface to store coordinates of key locations, for example ablation points.
Alternatively, devices incorporate conductive wires (or loop antenna) along the length of the device. Signals from these devices are displayed on top of previously acquired anatomic roadmaps (device localization only) or combined into the array of receivers used to update real-time CMR images. The device position or signal receiver can be color coded, to distinguish them from grey-scale background images of the anatomy. Devices displayed in this way are readily visible inside thick-slab projections resembling projection x-ray images showing devices in profile. Distinct signals can be imparted to the tip of devices so that it can be tracked in 3D, ideal for intracardiac applications. Alternatively, direct current applied to conductive elements along the device induce magnetic field inhomogeneities, disrupt local signal, and create dark impressions.
Unfortunately, the long conductive transmission wires used to connect catheter coils to the CMR transmitter or receiver system are susceptible to RF heating, which may damage neighboring tissue. Multiple approaches to reduce heating can be combined to make catheter devices safe, such as attaching circuitry to decouple and detune the transmission lines, intermittent chokes and transformers in the transmission lines, and insulation.
Another hybrid approach is to incorporate closed-loop receiver coils into stents or catheter devices, without connecting via transmission lines to the CMR system. These “inductively-coupled” devices resonate at predetermined geometric shapes and thereby amplify local RF signal. Unfortunately, inductively coupled catheter devices cannot easily be displayed in color during real-time CMR, as can other actively visualized catheter devices, and are best visualized at lower flip angles that compromise imaging.
The NHLBI active guidewire for cardiovascular applications incorporates a loop antenna for active visualization of the whole shaft and a separate active marker to distinguish the guidewire tip. The device also incorporates a fiberoptic temperature probe for continuous monitoring of RF-induced heating. The device appears in color overlay on real-time CMR images ( Fig. 47.6 ).

Finally, CMR pulse sequence techniques can reduce the energy deposited per image, and therefore heating, with an acceptable penalty in image quality. Such techniques include radial sampling, spiral sampling, and echo planar imaging (EPI), which use fewer excitation pulses and longer repetition times, variable flip angle sequences and parallel imaging. Techniques that reduce specific absorption rate at high fields, such as adaptive parallel transmission, may also prove helpful at lower fields to reduce device heating during CMR guided interventions.
Device Solutions for Cardiovascular Applications
In our experience, it has been useful to use a combination of both active and passive devices during complex interventional procedures, albeit in animal models. Real-time, active visualization of the device tip in 3D is desirable during certain procedures such as atrial transseptal puncture or myocardial wall injection. Similarly, active approaches prove useful while surveying devices for common failure modes such as buckling, looping, or kinking. Passive approaches unencumbered by electrical hardware attachments are sufficient for simple procedures such as placing introducer sheaths that typically do not move once positioned. More importantly, combining passive devices (such as balloons filled with dilute Gd-DTPA) with active devices (such as an active guidewire) generates wonderful and useful images ( Fig. 47.5 ).
Catheters for iCMR are usually manipulated manually by the operator in a similar fashion to a standard x-ray catheterization laboratory. However, by applying current to wire coils wound at the tip of a catheter, it is possible to remotely steer a catheter under real-time iCMR guidance. Using this approach, Hetts et al. developed an endovascular catheter system and demonstrated comparable procedure times for navigation through a vascular phantom with MRI and conventional x-ray guidance. The same group used these catheters to perform renal artery catheterization and embolization in swine. However, it is questionable whether remote-controlled catheters really decrease procedure time in experienced hands.
Applications
Extra-Anatomic Bypass
Advances in transcatheter therapy for many congenital cardiovascular conditions have reduced the need for invasive open surgery. As noted earlier, children may be particularly sensitive to the harmful effects of x-ray radiation. Children with complex congenital cardiovascular disease often undergo multiple x-ray procedures and have greater cumulative radiation exposure and greater lifetime risk of radiation-induced malignancy. Radiation-sparing procedural guidance with CMR is attractive and forms the basis for a number of real-time CMR guided research applications.
Full visualization of all anatomic structures and soft tissue may facilitate complex procedures that involve crossing anatomic boundaries and connecting remote vascular structures. Using a double-doughnut CMR configuration, Kee et al. conducted preclinical and clinical transjugular intrahepatic porto-systemic shunt (TIPS) procedures. Even in this proof-of-concept experiment, MRI reduced the number of transhepatic needle punctures compared with historical controls. Arepally et al. created an elegant catheter-based mesocaval shunt outside the liver capsule.
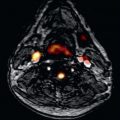
Ratnayaka et al. developed the preclinical capability to use catheters instead of surgery for one of the three staged surgical repairs to palliate children with single ventricle physiology. They demonstrated real-time iCMR guided cavopulmonary shunt using active needles, Gd-filled balloon-mounted covered stents, and custom-built endografts ( Fig. 47.7 ). Although theoretically feasible using x-ray fluoroscopy and contrast angiography, CMR enables visualization of origin and destination vascular structures in orthogonal planes as well as all interposed tissues. Furthermore, CMR enables immediate identification of important complications, for example, pericardial effusion or vascular dissection. The ultimate goal is to develop percutaneous approaches to replace some of the open-chest surgeries that children with complex congenital heart disease will require over their lifetime.

Endomyocardial Biopsy
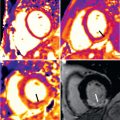
Endomyocardial biopsy remains the preferred diagnostic test in patients with unexplained cardiomyopathy and in heart transplant recipients with suspected rejection. Yet x-ray guided biopsy is essentially a blind procedure because neither important structures, such as valves or chordae, nor the target endocardium can be visualized. In pathologies affecting the heart in a nonuniform distribution, biopsy can often provide inconclusive results because a negative specimen may simply reflect that a nonaffected part of the heart was sampled. In contrast, CMR offers a number of tools for advanced tissue characterization that may help distinguish normal from abnormal myocardium, for example, late Gd enhancement (LGE) and T1 mapping. Real-time iCMR guided endomyocardial biopsy could potentially enhance diagnostic yield and safety by targeting abnormal myocardium and avoiding vulnerable structures. Both passive-visualization and active-visualization bioptomes have been developed for in vitro and preclinical testing. We developed an animal model of focal myocardial pathology and demonstrated significantly higher diagnostic yield with iCMR versus x-ray guided biopsy ( Fig. 47.8 ).