LEARNING OBJECTIVES
1. Ductography
• Indications for procedure
• How is the procedure done?
• Findings and differential considerations
• Diagnostic versus preoperative ductography
• When should wire localization be used in conjunction with preoperative ductography?
2. Cyst aspiration and pneumocystography
• Indications for cyst aspiration
• Indications for pneumocystography
• Procedures
• Complex cystic masses: management
3. Needle biopsies
• Indications for imaging-guided biopsies
• Ultrasound, stereotactic, and MRI guidance
• Clip placement
• Post procedure patient management
• Radiology–pathology concordance and patient management
4. Preoperative wire localizations
• Indications
• Approaches
5. Specimen evaluation
• Radiography
• Sonography
6. Paraffin block radiography
• Indications
• Orthogonal imaging
DUCTOGRAPHY
The evaluation and management of patients presenting with nipple discharge is variable among clinicians. Commonly, the prolactin level is checked and some of the discharge is submitted for cytological evaluation; if the results are normal, the patient usually undergoes no further workup unless the discharge is bloody. Patients with bloody nipple discharge are referred to a surgeon and may undergo a subareolar duct excision. The assumptions made with these approaches need to be challenged as outlined in Table 12.1.
Although ductography is not a perfect test, it can help address some of the issues raised by the assumptions presented in Table 12.1. The presence, location, and extent of lesions can be demonstrated in many patients with spontaneous nipple discharge. The location of the duct containing the lesion and its distribution in the breast can be established preoperatively. When findings consistent with duct ectasia or fibrocystic changes are diagnosed, surgery may be averted. Lastly, if preoperative ductography is done using a methylene blue:contrast (1:1) combination, the contrast allows us to verify that we cannulated the abnormal duct; the methylene blue stains the duct; so it is easy to identify by the surgeon intra-operatively and the pathologist at the time the specimen is processed. This helps assure that the lesion is localized, excised, and evaluated histologically.
Table 12.1 COMMON ASSUMPTIONS IN WOMEN WITH NIPPLE DISCHARGE
Assumption | Fact |
1. Negative cytology results reliably exclude pathology | Negative cytology does not reliably exclude significant pathology |
2. Only bloody nipple discharge is significant | DCIS can present with serous or clear, heme occult negative nipple dischargea |
3. The location and extent of a lesion in the duct can be reliably identified intra-operatively so that the dissection is extended as needed to include, but not transect, the lesion | Not all papillomas are close to the subareolar area, and when DCIS is present, it is often extensive and not subareolar in location |
4. The pathologist can reliably identify the location of the lesion in the specimen submitted for histologic processing | Staining of the duct with methylene blue facilitates intra-operative identification of the duct for excision by the surgeon and during processing of the specimen by the pathologist |
aHow a patient notices the discharge is more important than the character of the discharge. Expressed nipple discharge is usually physiologic. Spontaneous nipple discharge needs to be evaluated regardless of its appearance. | |
Clinically, patients with intraductal lesions may present describing nipple discharge. When asked how they notice the discharge, patients invariably provide one (or all) of three histories. They notice dark spots on their bra cups or spots on their nightclothes, or after taking a hot bath or shower they notice their nipple is dripping. If the discharge is only expressed after vigorous manipulation of the breast and nipple, the likelihood of finding an intraductal lesion is low and ductography is not indicated. Patients should be discouraged from trying to express nipple discharge. As part of breast self-examination, they should be instructed to get into the habit of checking their bra cups for dark brown spots.
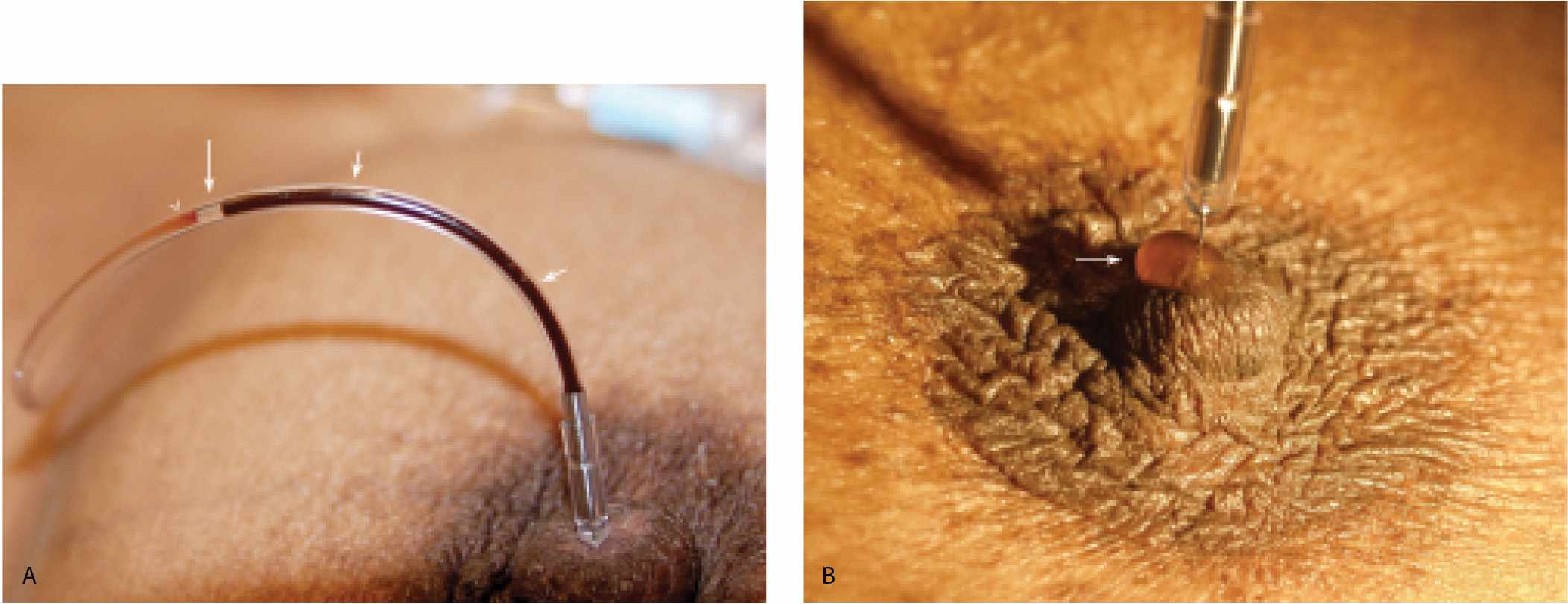
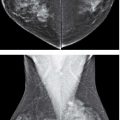
Ductography is a safe, easy, and simple contrast evaluation of a lactiferous duct (1–4). The only relative contraindication to ductography is the presence of mastitis and no significant complications have been described with the water-soluble contrast agents currently in use. Ductography is used to evaluate women presenting with spontaneous nipple discharge, regardless of the character of the discharge (Fig. 12.1). Adequate lighting and magnification of the nipple are helpful in identifying the secreting duct opening. Full-strength iodinated contrast material is drawn into a 3-mL Luer lock syringe. A 30G blunt-tip sialography needle with attached tubing is screwed onto the Luer lock syringe, making sure that the connection is tight so that air bubbles are not drawn into the syringe. Contrast material is run through the tubing until all air bubbles are removed from the system. Topical anesthesia and dilators are not needed for this procedure.
Although we place the patient in a supine position with the ipsilateral arm placed above her head, others do these procedures with the patient sitting up. The nipple is inspected for the presence of crusting or a minimally prominent, erythematous and patulous duct opening. After the visual inspection of the nipple, an alcohol wipe is used to swab the nipple and remove any keratin plugs that may obstruct the duct opening. Next, the breast is examined. A “trigger point” may be identified in some patients (5). When this “trigger point” is compressed, nipple discharge is obtained. As you move away from this point, the discharge stops. In patients with an intraductal lesion, the discharge is commonly copious and “projectile.”
If you identify a trigger point, use it to elicit small amounts of discharge. Elicit just enough to moisten and glisten the duct opening. If a larger amount of discharge is obtained, other openings are flooded, precluding correct identification of the offending duct. With a clear idea of which opening the discharge is coming from, angle the cannula and place the tip in the duct opening. After the cannula is engaged in this manner, straighten it so that the cannula “falls” into the duct to the level of the hub. After cannulation, take a few seconds to observe the tubing. Since the tubing is now in a closed system with the cannulated duct, duct contents can sometimes be seen refluxing back into the tubing (Fig. 12.2A). If this does not happen, inject a small amount of the contrast and look at the nipple. As intraductal fluid is displaced by contrast, some of the fluid may come out to form a drop around the cannula (Fig. 12.2B). When either of these observations is made, you have some assurance that you cannulated the potentially abnormal duct.
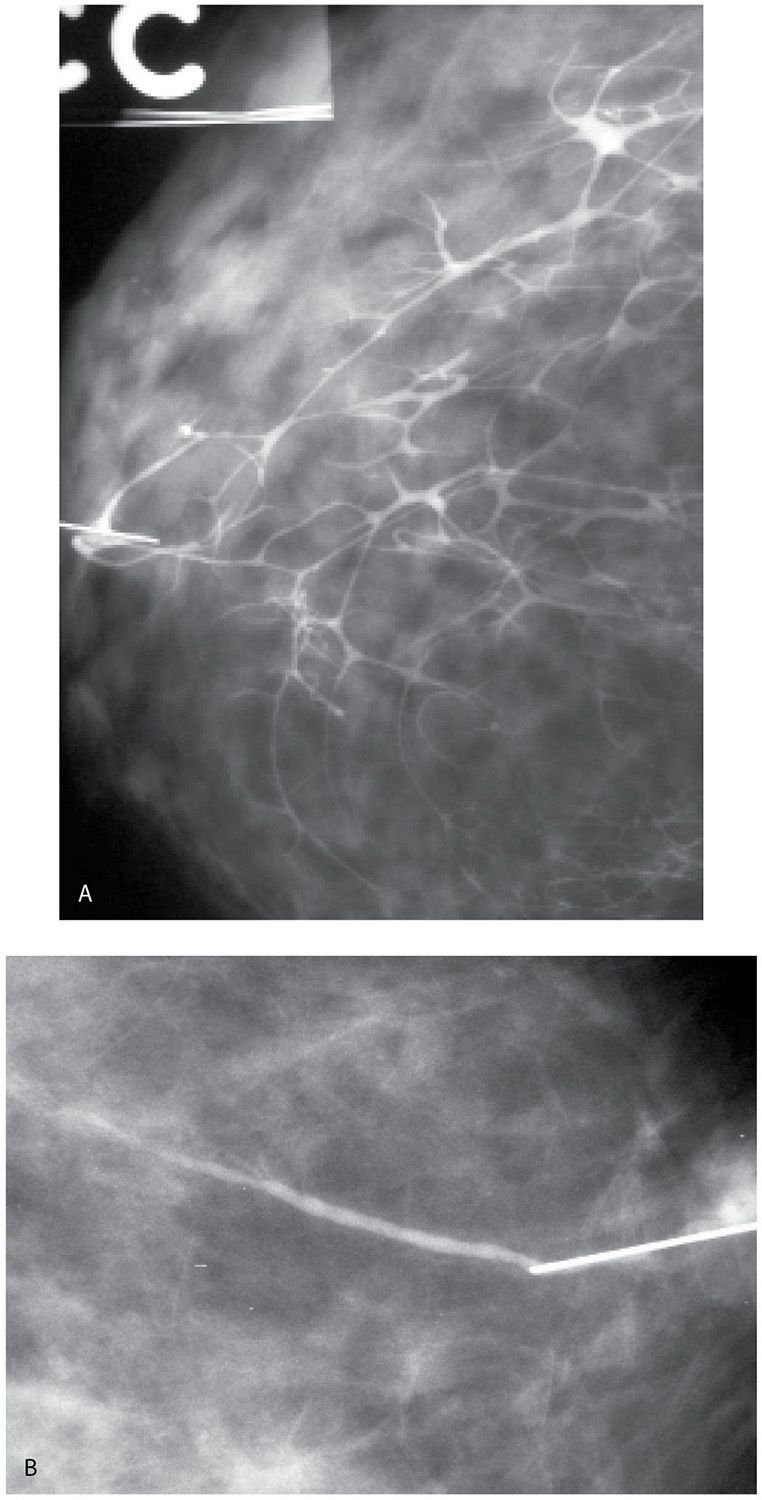
A small amount of contrast (0.2–0.4 mL) is injected initially and the cannula is taped on the nipple. Injecting larger amounts of contrast at the onset may obscure small lesions (Fig. 12.3) or those that are close to the nipple. Full paddle magnification views in the craniocaudal (CC) and 90-degree lateromedial (LM) projections are obtained. After a review of these initial images, additional contrast can be injected, as needed to opacify the duct proximally (Fig. 12.3).
Little is known about the ductographic appearance of “normal” ducts. There seems to be considerable variation in length, amount of branching, distribution, and caliber (Fig. 12.4). Occasionally, a contained contrast blush is seen, the overall appearance of which suggests the possibility of lobular opacification (Fig. 12.3B, C). We use the sialography cannula as an internal measure of duct caliber. Arbitrarily, we define normal duct caliber as up to three times the width of the cannula.
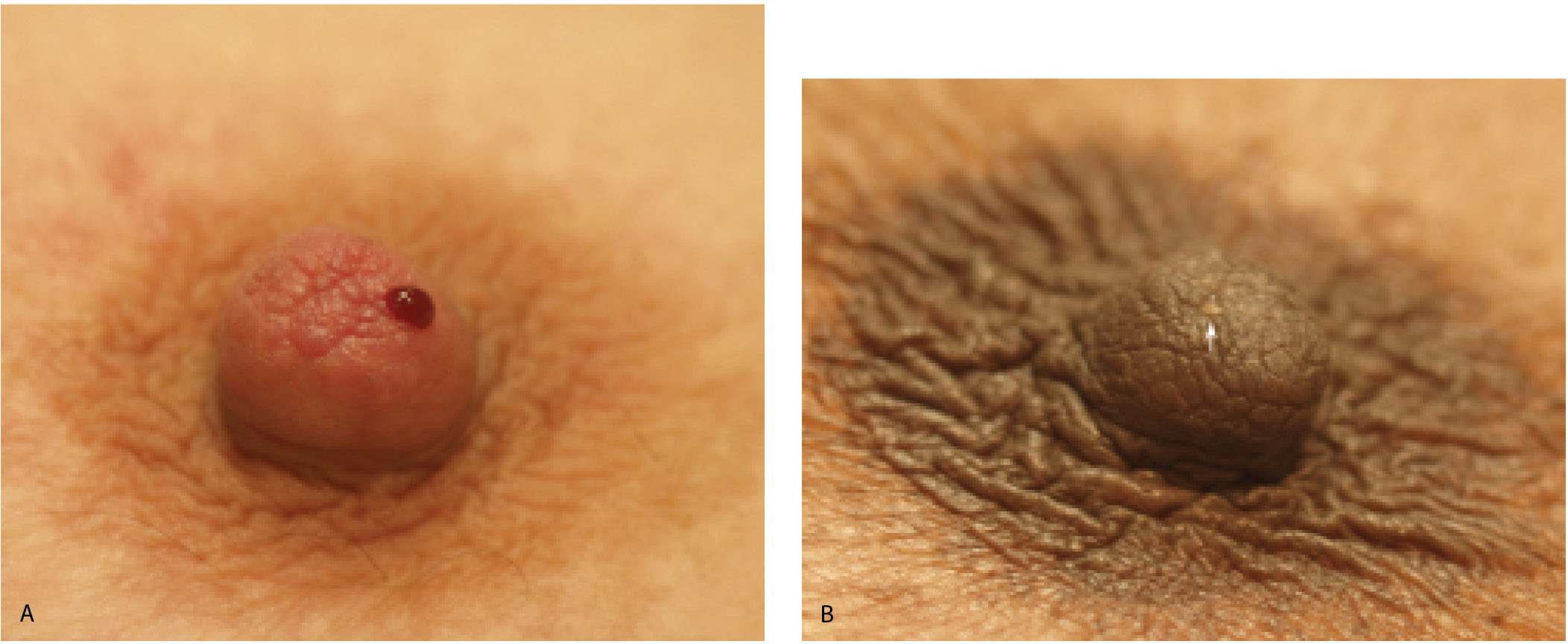
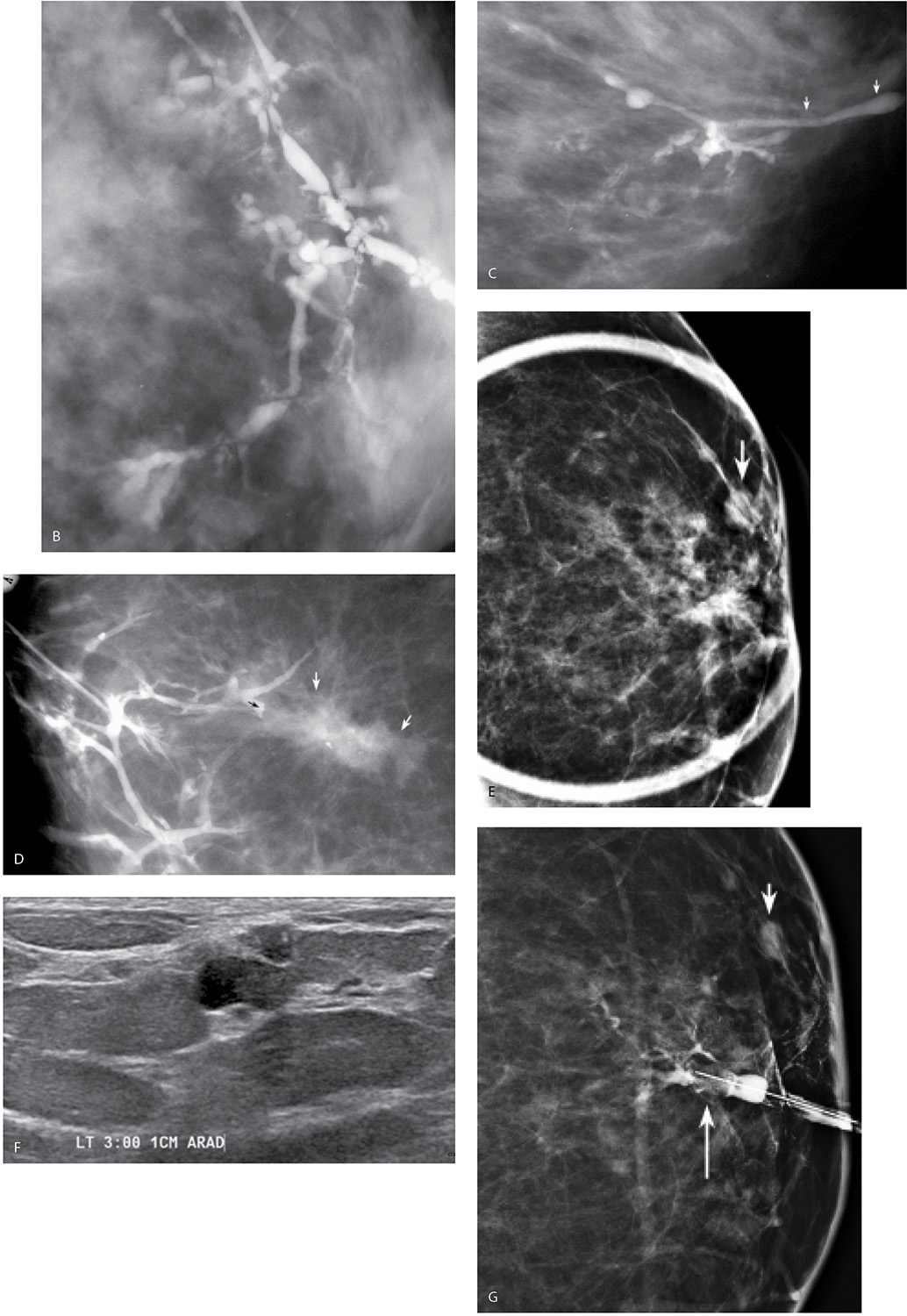
FIG. 12.1 • Nipple discharge. A: Bloody nipple discharge arising from a single duct opening. B: Clear nipple discharge (arrow) arising from a single duct opening. If the patient presents with nipple discharge, it is important to establish if it is expressed or spontaneous by asking the patient how she notices the discharge. If spontaneous, the character (bloody, clear, serous, etc.) of the discharge does not dissuade us from evaluating the patient with ductography. Breast cancers can present with clear or serous, heme occult negative discharge. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
FIG. 12.2 • Establishing accurate cannulation. A: Cannula in the duct to the hub. Immediately after you cannulate the duct, look at the tubing for a few seconds before injecting contrast. The tubing is now in a closed system with the cannulated duct so duct contents can sometimes be seen refluxing into the tubing. If this is seen, it confirms cannulation of the duct producing the discharge. In this patient, the refluxing material is bloody (short arrows). An air bubble (long arrow) is present in the tubing. Also note intermingling of bloody duct contents with the contrast (arrow head) in the tubing. When the discharge is clear or serous, mixing of the duct contents with the contrast can be seen occurring in the tubing. B: Duct contents forming a serosanguineous droplet around the cannula. If duct contents do not reflux into the tubing, duct contents can sometimes be seen forming a droplet (arrow) around the cannula as contrast is injected. The contrast displaces duct contents. When this is seen, it confirms cannulation of the duct producing the discharge. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
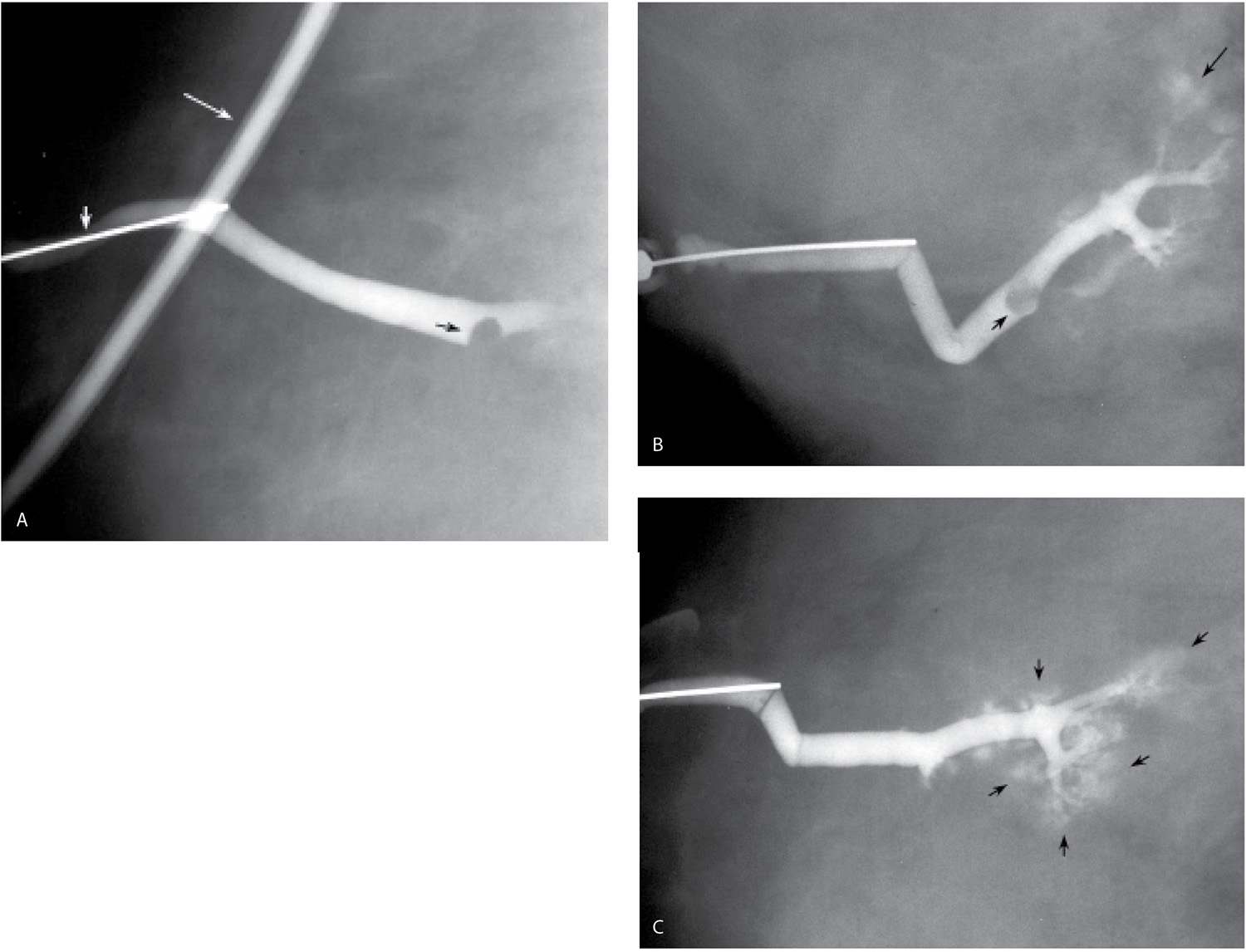
FIG. 12.3 • Masking of lesion, “lobular blushing,” papilloma. A: Initial films following injection of 0.2 mL of contrast demonstrate a filling defect (black arrow) reflecting a papilloma. No proximal opacification of the duct is noted. Cannula (short white arrow) is noted in the duct. The tubing with contrast (long white arrow) should be moved out of the field of view by the technologist. B: Following the injection of an additional 0.2 mL of contrast, the filling defect (short black arrow) is still seen. Branches of the duct are now filling with contrast and there is some “blushing” (long black arrow) possibly reflecting contrast in lobular units. C: An additional 0.2 mL of contrast is injected. The lesion is no longer seen secondary to the amount of contrast injected. Contrast “blushing” is increasing (arrows). Small lesions, and lesions that are close to the nipple, can be obscured if too much contrast is injected initially. A small amount is injected initially. If the cannula is left in place, it is easy to inject additional contrast as needed to evaluate the duct proximally. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
FIG. 12.4 • “Normal” ducts. A: If we use the cannula as an internal reference, this duct is normal in caliber. This duct demonstrates a wide area of drainage with a moderate amount of branching. No focal abnormality is apparent. B: Different patient. This duct has no detectable branch points and its distribution is limited in the breast. Normal caliber. As demonstrated in these two different patients, what we presume to be normal ducts are variable in length, branch pattern, and distribution in the tissue. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
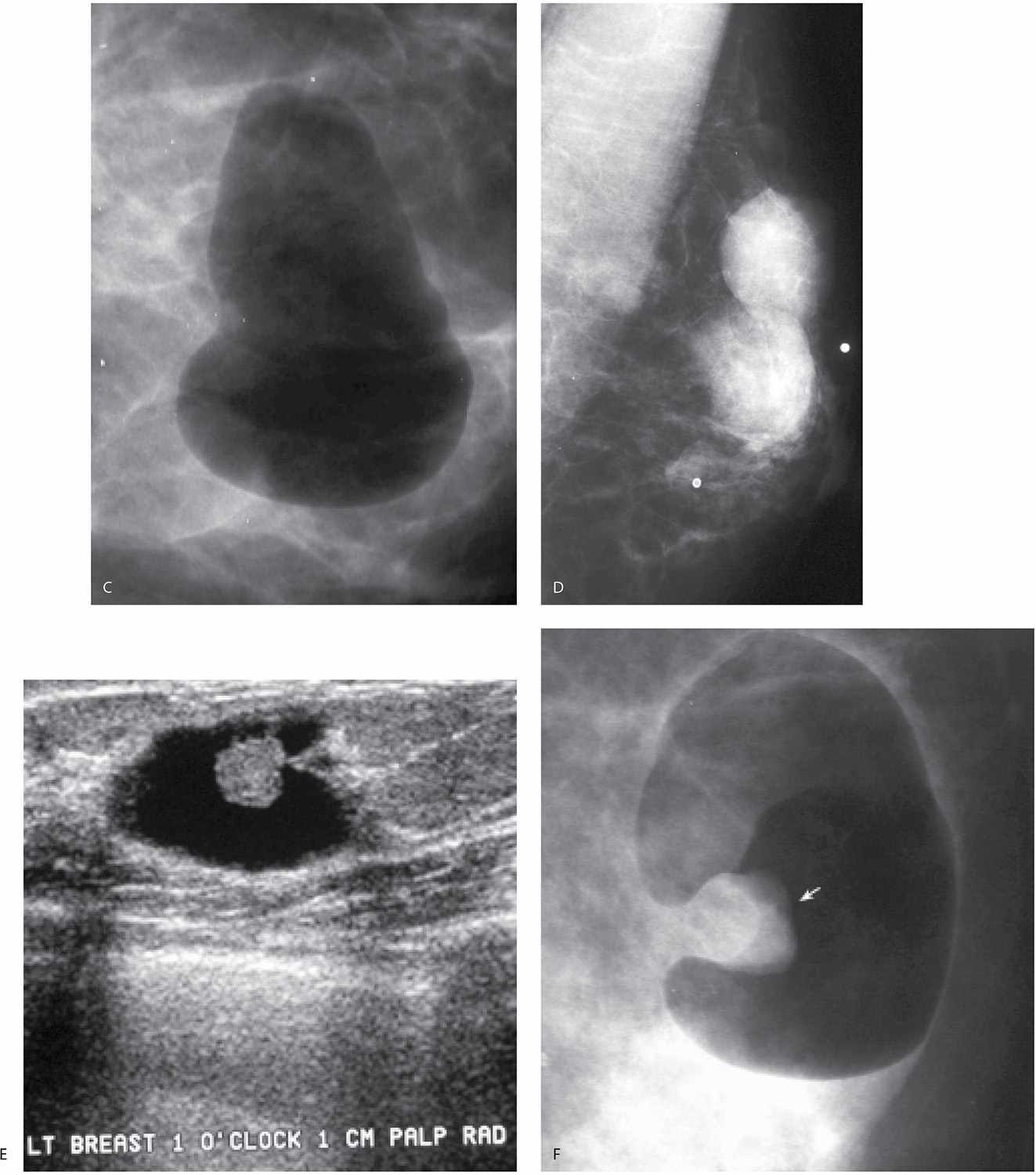
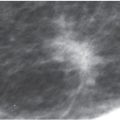
Solitary papillomas are diagnosed in approximately 50% of women presenting with spontaneous nipple discharge (1–7). Papillomas are most commonly found within dilated ducts in a subareolar location (see Figs. 9.33 and 9.34A, B). Although the entire duct may be dilated in these patients, the segment of duct between the lesion and the nipple is most commonly involved (Fig. 12.5A). Papillomas, however, can occur anywhere in the duct, and duct dilatation is not always present. Filling defects (Fig. 12.5B, C; also see Fig. 9.33), obstruction of the main duct (Fig. 12.5A) or a branch, and, less commonly, wall irregularity (Fig. 12.5D) are ductographic findings in patients with papillomas. In some patients, contrast can be seen pooling irregularly in the interstices of the lesion. The lesions sometime appear to expand and disrupt the integrity of the duct; however, when excised, the wall of these ducts is intact (Fig. 12.6). In patients with fibrocystic changes, the discharge is often green in color (7). Ductography findings include connection with one or several cysts (Fig. 12.7) or diffuse wall irregularities. Thick white pasty discharge (Fig. 12.8A) may be seen in patients with underlying duct ectasia as the cause of nipple discharge (7). These ducts are dilated in the subareolar region and often change abruptly in caliber as the duct courses proximally (Fig. 12.8B).
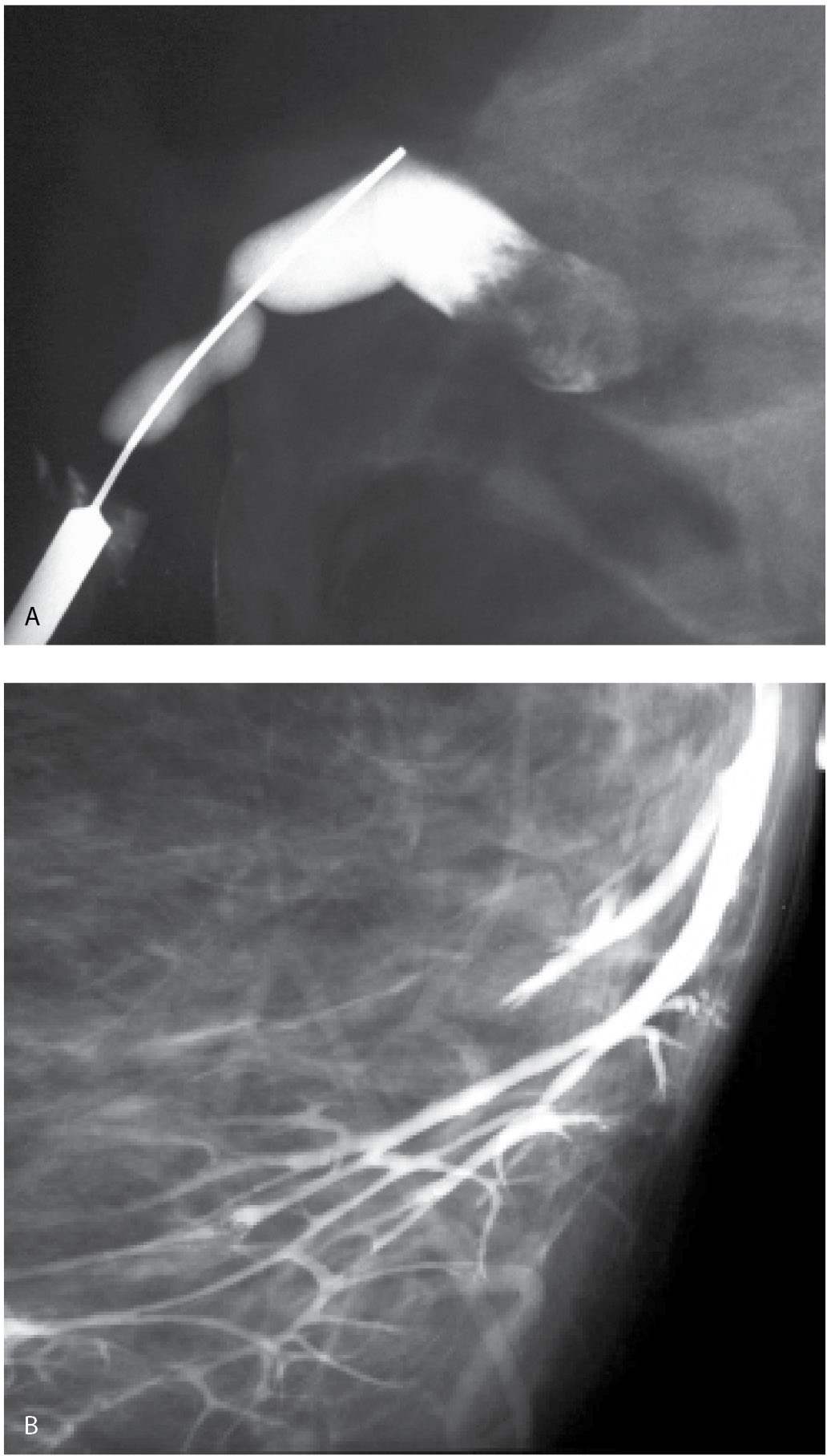
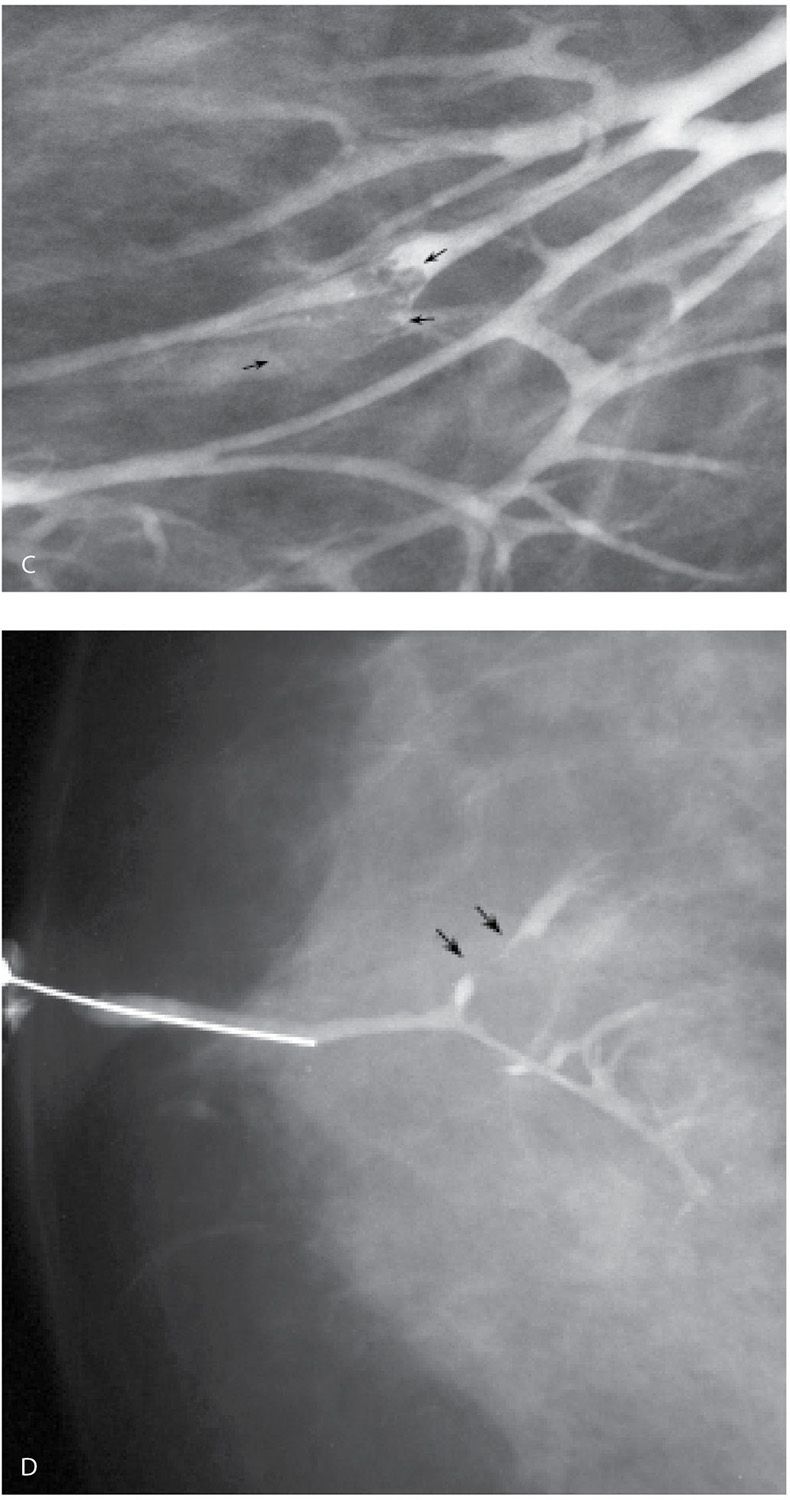
FIG. 12.5 • Papillomas. A: A lesion is present obstructing the cannulated duct close to the nipple. The irregular interface at the obstructing site reflects contrast pooling in the interstices of the papilloma. The duct between the lesion and the nipple is dilated (compare to cannula). The subareolar location of this lesion in a distended duct is common with papillomas. B: Different patient. Opacified duct is moderately dilated. Do you see the lesion? In spite of the magnification technique, some of these lesions can be difficult to identify, and close evaluation of all branches is important. Imagine the limitations in evaluating ducts and detecting lesions without the use of magnification. C: Photographic coning to the area of the lesion demonstrates a filling defect (arrows) in the opacified duct shown in part B. The edges of the lesion are irregular consistent with contrast pooling in the interstices of the papilloma. Notice the distance from the nipple to the lesion. Although papillomas are often subareolar in location not all of them are. Without knowing the location of this lesion preoperatively, do you think the surgeon would extend his/her dissection to this point? Even if they did, can we be sure the pathologist would know where to look for the lesion? D: Different patient. The caliber of opacified duct is normal. An area of persistent narrowing and irregularity is noted involving a side branch of the duct (arrows). Although ductal carcinoma in situ is suspected in this patient, a papilloma is diagnosed after wire localization and excision. The findings of papillomas and ductal carcinoma in situ on ductography overlap therein the need for excisional biopsy. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
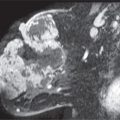
Lastly, breast cancer can present with spontaneous nipple discharge (1–7). In this group of patients, three subgroups should be considered: (i) patients with ductal carcinoma in situ (DCIS) arising in a papilloma; in this group the opacified duct is often dilated and one or multiple intraductal lesions are identified (Fig. 12.9A); (ii) patients with DCIS in whom the duct is not usually dilated and the findings involve the opacified duct diffusely (Fig. 12.9B, C); and (iii) patients with invasive ductal carcinoma in whom a mass may be identified on the mammogram (Fig. 12.9D). Most of the patients with breast cancer who present with nipple discharge in groups (i) and (ii) have normal mammograms. Of all patients presenting with spontaneous nipple discharge, 5% to 8% will be diagnosed with breast cancer and it is commonly DCIS. A small number of patients presenting with spontaneous nipple discharge have a finding suggestive of cancer (e.g., malignant-type calcifications, mass with indistinct or spiculated margins) identified on the mammogram (Fig. 12.9D). In these patients, we do not assume that the nipple discharge is necessarily related to the mammographic finding. Until a relationship is established, these may represent synchronous yet unrelated processes (Fig. 12.9E–G). The findings on ductography in women with breast cancer overlap those described for papillomas. They include one or multiple filling defects, obstruction of the duct, and duct wall irregularity. Less common signs of cancer on ductography include contrast extravasation and displacement of the opacified duct (3).
FIG. 12.6 • Papilloma, expanding and distorting the duct. This lesion is obstructing the duct. Contrast is seen seemingly outside the confines of the duct (black arrows) but this is actually contrast in the interstices of the lesion and the duct itself is normal. If we use the cannula (white arrow) as an internal reference, this duct is dilated. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
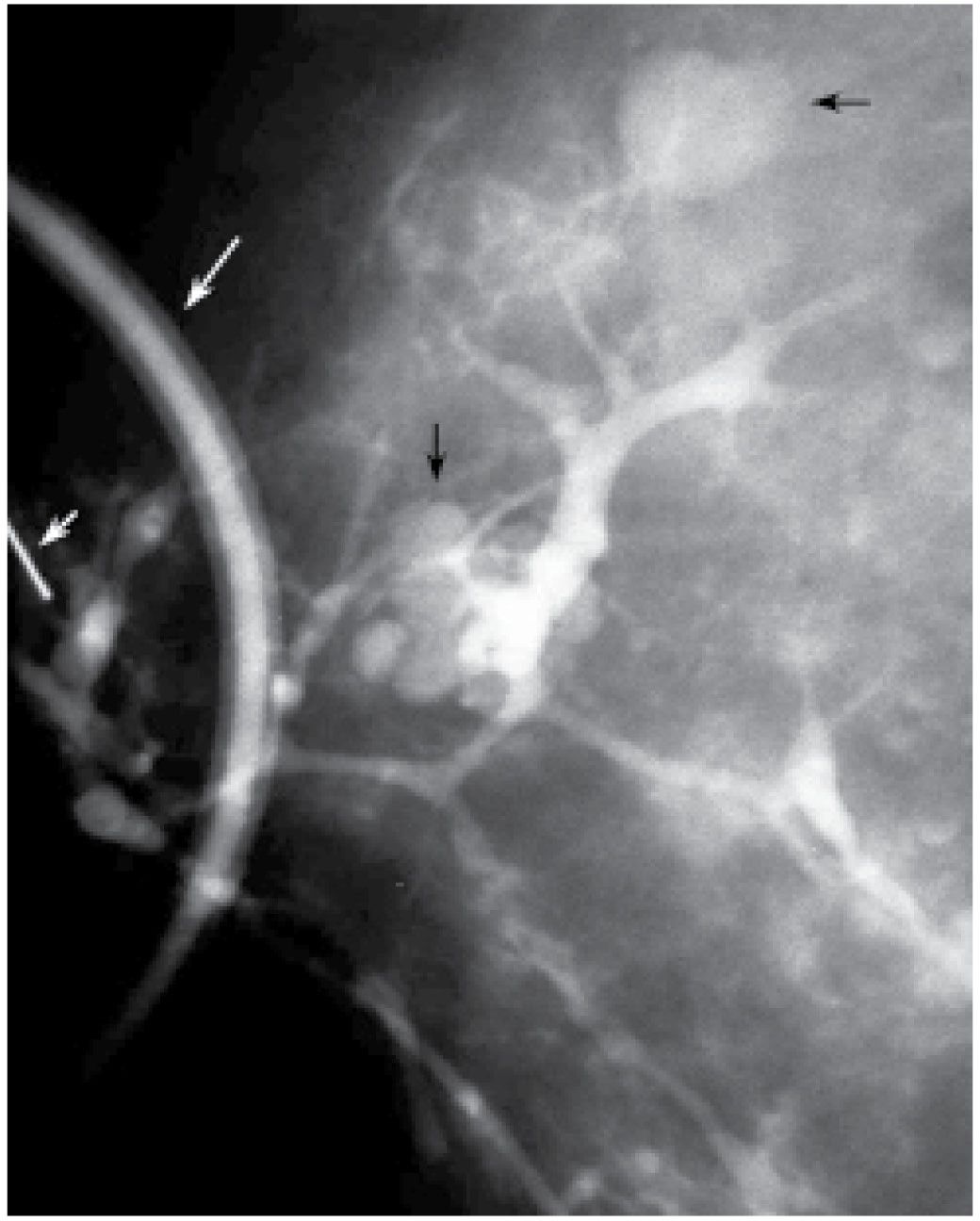
FIG. 12.7 • Fibrocystic changes, connection to cysts. Contrast is seen opacifying multiple cysts (black arrows). A portion of the contrast containing tubing (long white arrow) used for the ductogram is seen superimposed on the breast. Ideally, the technologist moves the tubing away from the field of view. A small portion of the cannula (short white arrow) is seen at the edge of the image. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
FIG. 12.8 • Duct ectasia. A: Thick white discharge (sometimes malodorous) is seen commonly in women with duct ectasia. B: Different patient. The duct is dilated in the subareolar area with an abrupt change in caliber. The more proximal branches of the duct assume a normal caliber. No focal finding is identified. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
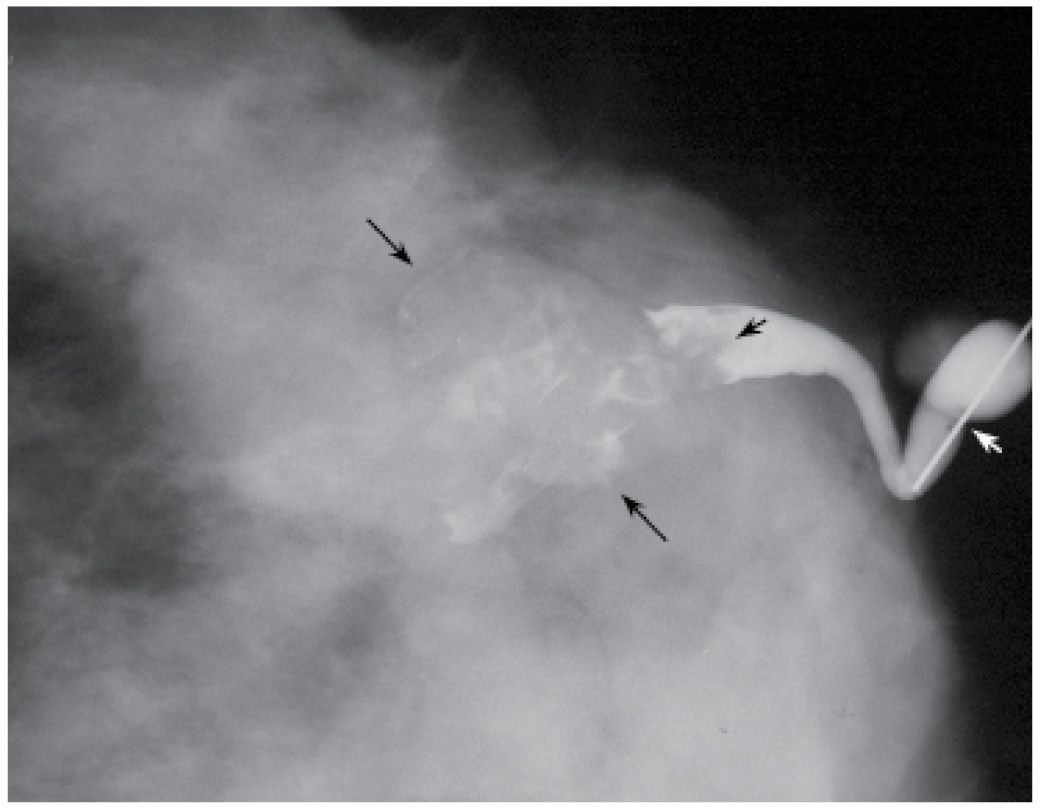
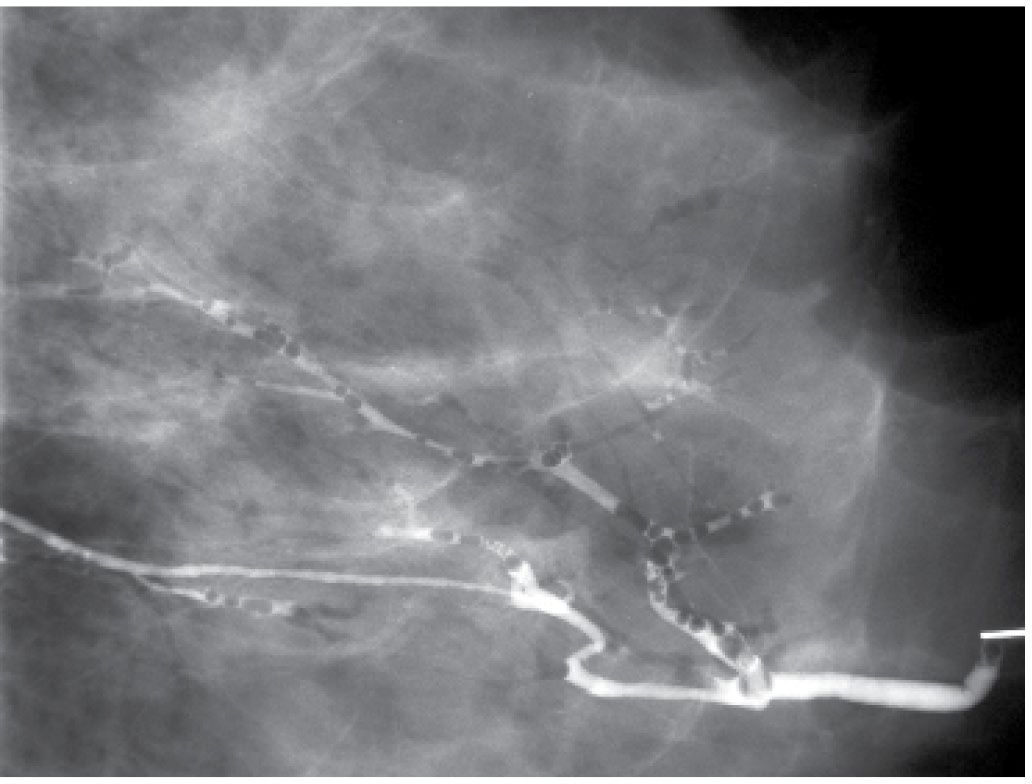
Potential pitfalls include masking of small lesions, or those close to the nipple, if too much contrast is injected at the onset (Fig. 12.3). If masking of a lesion close to the nipple is suspected, a follow-up image can be done after the cannula is removed. Air bubbles may rarely be mistaken for a lesion. Air bubbles, however, are well defined, round, lucent, and change in position between images (Fig. 12.10). Rarely in some patients, an extensive amount of air outlines portions of the duct. This is probably not related to the contrast injection; however, the cause is not usually established. Duct perforation with contrast extravasation is uncommon. It is not easy to perforate a normal duct. It requires a certain amount of force, is painful and the patient describes a burning sensation as soon as you start injecting contrast. In attempting to distend small ducts, contrast extravasation proximally in side branches of the duct and opacification of lymphatic channels can sometimes be seen (Fig. 12.11). Patients with proximal extravasation tolerate the injection initially, but describe a burning sensation after a few drops of contrast have been injected.
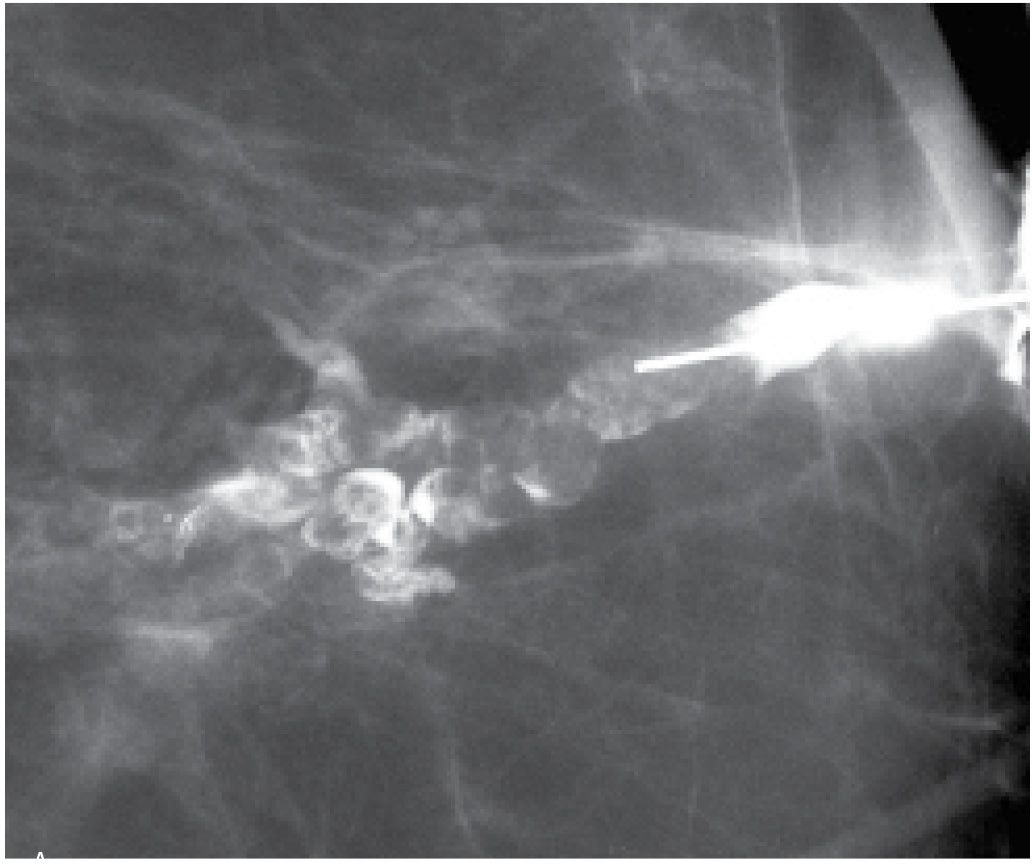
FIG. 12.9 • Ductal carcinoma. A: Intraductal lesion with interstices outlined by contrast. The duct is dilated between the lesion and the nipple. A papilloma with associated and adjacent ductal carcinoma in situ (low to intermediate nuclear grade with no central necrosis) is diagnosed histologically. B: Different patient. Magnified craniocaudal view of the anterior aspect of the left breast in a 42-year-old patient presenting with spontaneous nipple discharge. Her mammogram (not shown) is normal. A diffusely abnormal duct extends proximally into the upper central and lateral portions of the left breast. Multiple areas of narrowing and sacculation are identified, as are abrupt rounded terminations of the duct. Extensive ductal carcinoma in situ is described histologically. C: Different patient. The segment of the duct closest to the nipple (arrows) is normal. The remainder of the duct is diffusely abnormal with areas of sacculation and narrowing. Ductal carcinoma in situ is reported histologically. D: An irregular mass with spiculated margins is apparent on the mammogram. A side branch of the opacified duct is obstructed (black arrow) with a meniscus noted at the obstruction site. This corresponds to the site of the mass (white arrows) on orthogonal views (only one view is shown) consistent with an invasive ductal carcinoma in a patient with nipple discharge and a mass on her mammogram. A–D (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.) E: Different patient with a mass in the left breast and nipple discharge. Spot compression view demonstrating a mass (arrow) in the left subareolar area laterally. F: Ultrasound. A complex cystic and solid mass is seen on ultrasound corresponding to the mass seen mammographically. G: Ductogram. An intraductal lesion obstructs the dilated duct (long arrow). You cannot assume that the nipple discharge and mass detected mammographically are related. In this patient, the mass (short arrow) is an atypical papilloma and the intraductal filling defect identified on ductography is ductal carcinoma in situ, intermediate nuclear grade. In patients presenting with mammographic findings and nipple discharge, do not assume the two are related; separate processes may underlie the findings.
FIG. 12.10 • Air bubbles. Many circumscribed, round and oval lucent filling defects are noted throughout the opacified duct. Obtaining a second image will usually show that these change in position. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
Rarely, pseudolesions may be seen on ductography. Diffuse wall abnormalities may be seen on the initial ductogram; yet at the time of the preoperative ductography, the findings are not confirmed (Fig. 12.12). Nonspecific fibrocystic changes are diagnosed in these patients. It is unclear why this is seen; however, it may represent debris or clots in the lumen of the duct. Lastly, false negative ductography secondary to the cannulation of the wrong duct occurs in approximately 15% of patients. Duct openings are closely apposed on the surface of the nipple so identifying the one with the discharge can be a challenge. If a patient presents with a classic history (e.g., dark spots on the bra cup) and physical examination (focal crusting on the nipple, identifiable trigger point with copious discharge arising from a single duct opening) suggestive of an intraductal lesion, a normal ductogram is not accepted. The patient is asked to return for a repeat study or ultrasound can be used in an attempt to localize the lesion.
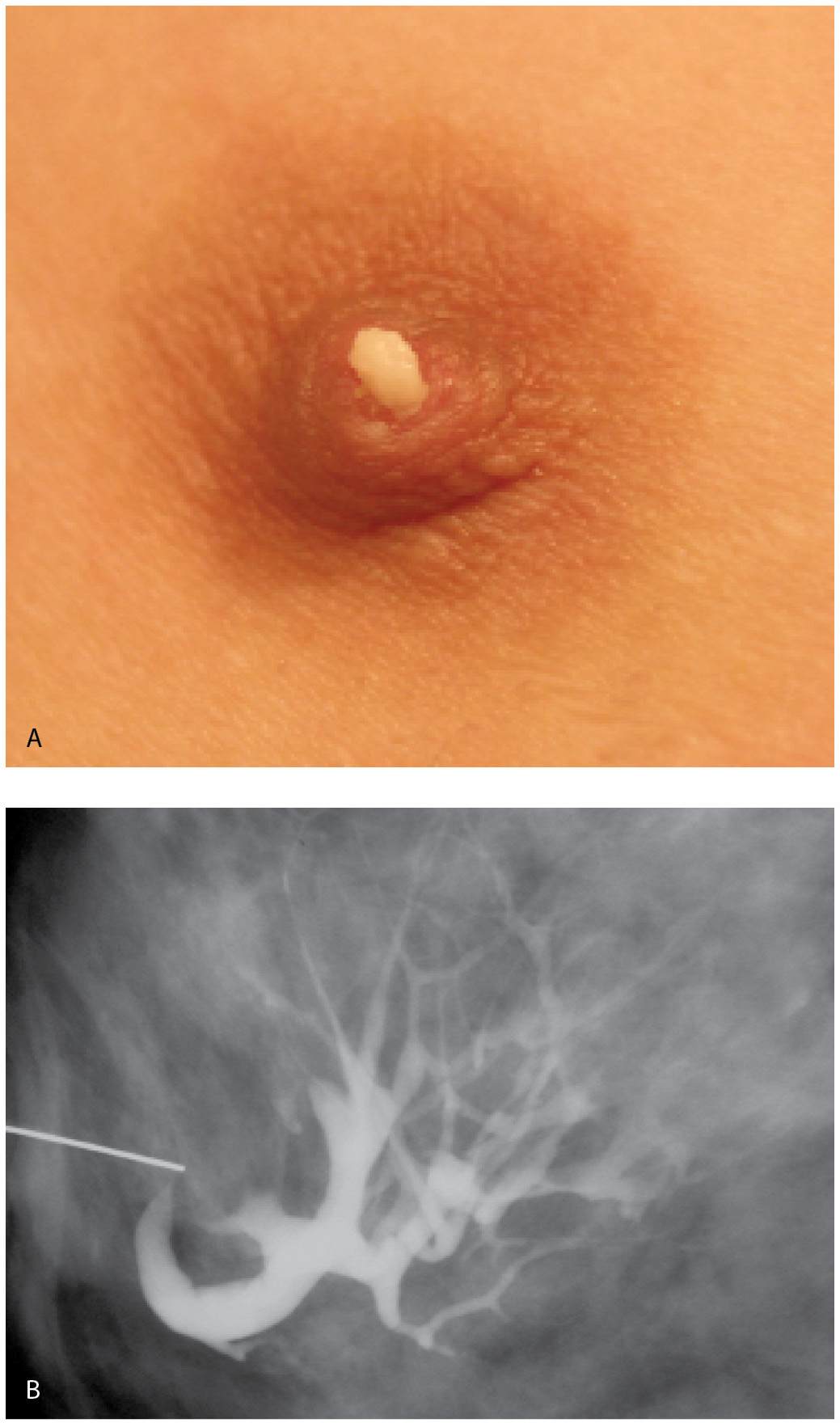
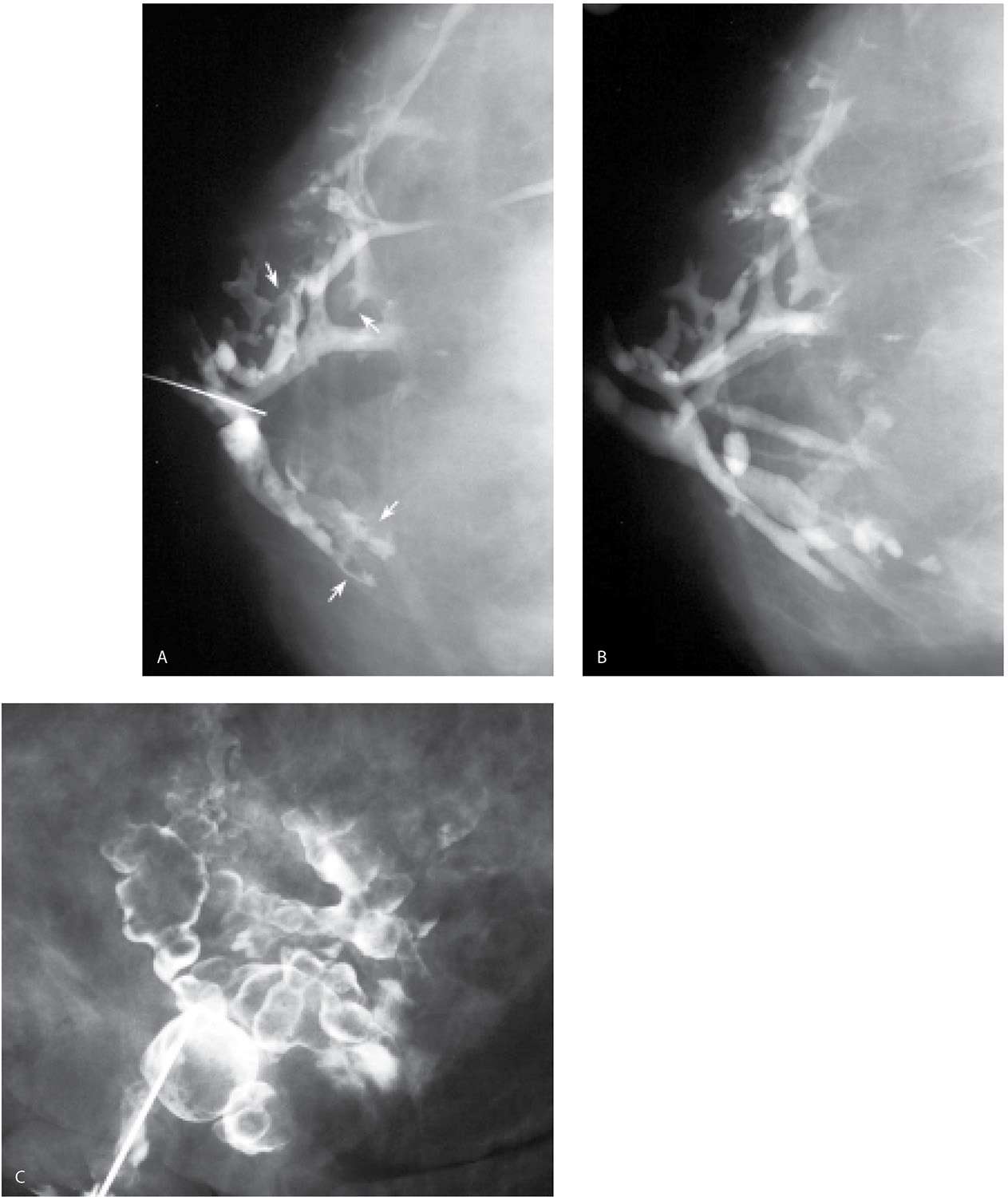
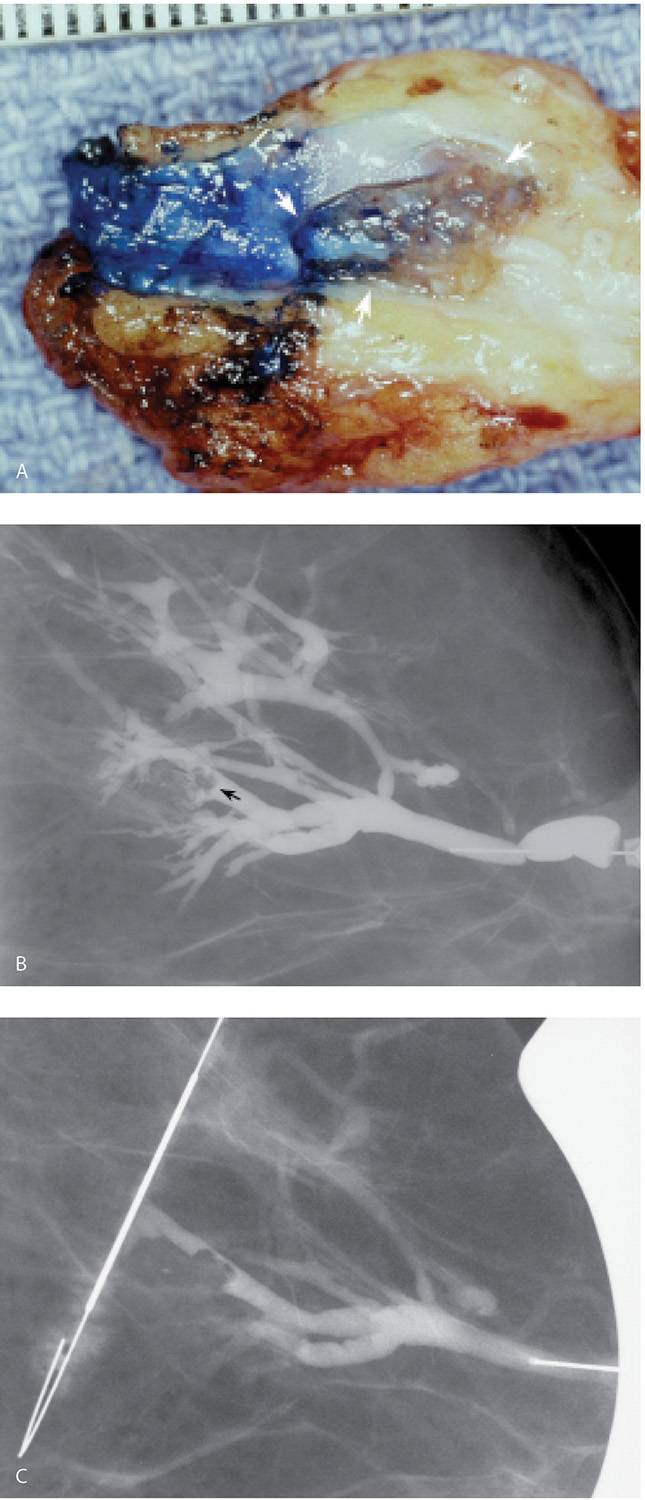
If an intraductal lesion is identified on ductography, excision is usually recommended. A preoperative ductography with a methylene blue:contrast combination (1:1) can be helpful to the surgeon intra operatively and assures the pathologist will process and evaluate the lesion (Fig. 12.13A). Occasionally, in women with lesions that are a distance from the nipple, or proximal to multiple branch points in the duct, a wire localization of the lesion is done using the ductogram to guide the wire localization procedure (Fig. 12.13B, C). In these patients, the ductogram is done with undiluted contrast material (e.g., no methylene blue is injected).
After the study is completed, the cannula is removed and the nipple is covered with gauze or a nursing pad. This prevents contrast material from leaking out onto the patient’s clothes. Many patients describe a significant reduction or complete cessation of the discharge for several weeks following the ductogram. It is unclear why this occurs but it is usually not a problem for the preoperative ductogram since discharge is still obtained when pressure is applied in the subareolar area.
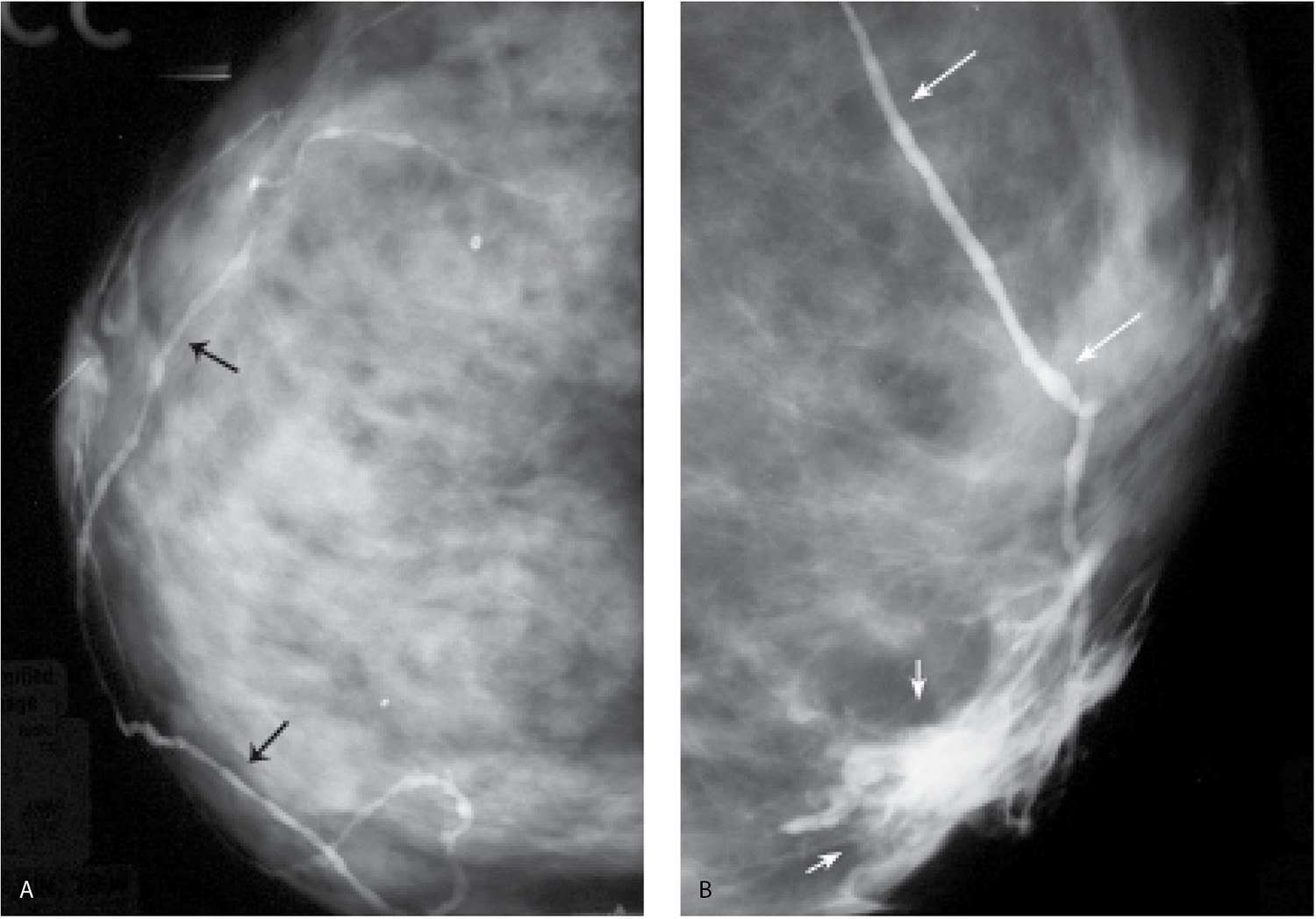
FIG. 12.11 • Opacified lymphatic channels. A: Tubular structures (arrows) are opacified with contrast. The random nonanatomic distribution of these structures (e.g., not ducts or vascular structures) is such that they are thought to represent lymphatic channels. B: Different patient. Proximal extravasation (short arrows) of contrast is noted resulting from an attempt to opacify additional branches in the cannulated duct. The patient describes burning as the extravasation occurs. A tubular structure (long arrows) in a nonanatomic distribution for a duct is opacified; this is thought to represent a lymphatic channel. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
FIG. 12.12 • Pseudo lesions. A: Diagnostic ductogram demonstrates a diffusely irregular duct with filling defects and wall irregularities (white arrows). Excision is recommended. B: Preoperative ductogram demonstrates a normal duct. Previously noted abnormalities are not reproduced. Nonspecific fibrocystic changes are reported histologically. A, B (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.) C: Different patient. Apparent filling defects diffusely involving a duct with focally dilated segments. It is unclear if these represent duct contents.
CYST ASPIRATION AND PNEUMOCYSTOGRAPHY
Aspiration of cysts is undertaken in three situations: (i) when there are associated symptoms (e.g., tenderness, burning), (ii) if observations are made on ultrasound such that the diagnosis of a cyst is in question, or (iii) at the patient’s request. As cysts enlarge and tension on the wall and surrounding tissue increases, patients may describe discomfort and tenderness. Some investigators have suggested that as cysts enlarge, some of the fluid escapes into the surrounding breast parenchyma eliciting an aseptic inflammatory response. Aspiration under these circumstances often relieves the symptoms (2,8,9).
FIG. 12.13 • Papillomas, preoperative ductogram with methylene blue or wire localization. A: Methylene blue stained duct is opened and the lesion (arrows) is identified in the duct and evaluated histologically. Preoperative ductograms are done using a contrast:methylene blue (1:1) combination. The contrast enables the radiologist to confirm cannulation of the abnormal duct. The surgeon uses the methylene blue to identify the duct intra-operatively. The methylene blue also stains the duct and, as in this patient, the leading edge of the lesion, for the pathologist. B: Different patient. Diagnostic ductogram demonstrates a filling defect (arrow) in a side branch of an arborized duct. C: The day of surgery, the ductogram is repeated and used to guide wire localization. Mid-portion of reinforced wire segment is just posterior to the intraductal lesion. B, C (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
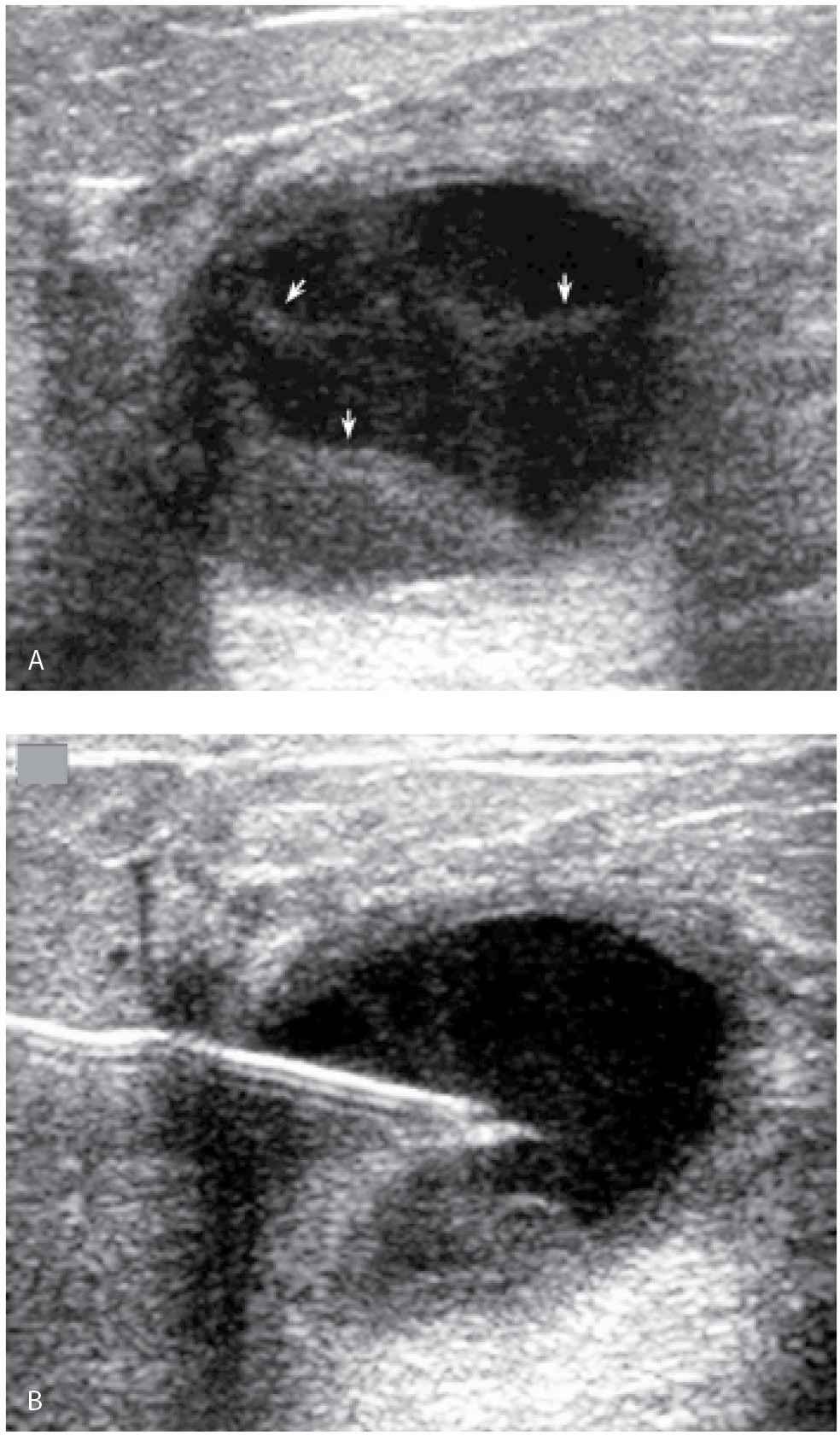
FIG. 12.14 • Cyst aspiration and pneumocystography. A: A seemingly complex cystic and solid mass with posterior acoustic enhancement is imaged corresponding to a lump described by the patient and a mass with obscured margins imaged on the mammogram. Internal echoes, apparent septations (arrows), and possible irregularity of the wall are noted. Aspiration and pneumocystography are done for diagnostic purposes. B: Needle with tip in the center of the mass. The aspiration is monitored during real time and the echoes are seen being aspirated into the needle. No residual abnormality is seen on ultrasound post aspiration. After the contents are aspirated, 50% of the volume of fluid aspirated is replaced with air. C: Ninety-degree lateral (LM) spot compression magnification view is done following the injection of air. A smooth-walled cyst cavity is seen with no intracystic or mural component. This is confirmed on orthogonal projections (craniocaudal spot compression view not shown). The findings are consistent with a cyst, possibly aseptically inflamed (given initial mural irregularities) warranting no further intervention or follow-up. D: Different patient. Two masses with circumscribed and indistinct margins are apparent mammographically in a 60-year-old patient presenting with a “lump.” The metallic BB marks the site of the palpable finding. E: Ultrasound. A complex cystic and solid mass (predominantly solid with a solid component) is imaged correlating to the palpable finding. The other mass seen mammographically is a cyst (not shown). Aspiration and pneumocystography are done. The fluid is serous in appearance. “Findings consistent with cyst contents” are reported cytologically (the pathologist is aware that a papillary lesion is suspected). F: Pneumocystogram image demonstrates intracystic mass (arrow). The remainder of the cavity has a smooth wall. Excisional biopsy following ultrasound-guided wire localization is done (see Fig. 12.41). In our experience, fluid reaccumulates (sometimes quickly) in patients with neoplastic (intracystic or mural) lesions – see Fig. 12.41 for the US on this patient on the day of the localization procedure and Fig. 12.18 for images on another patient. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
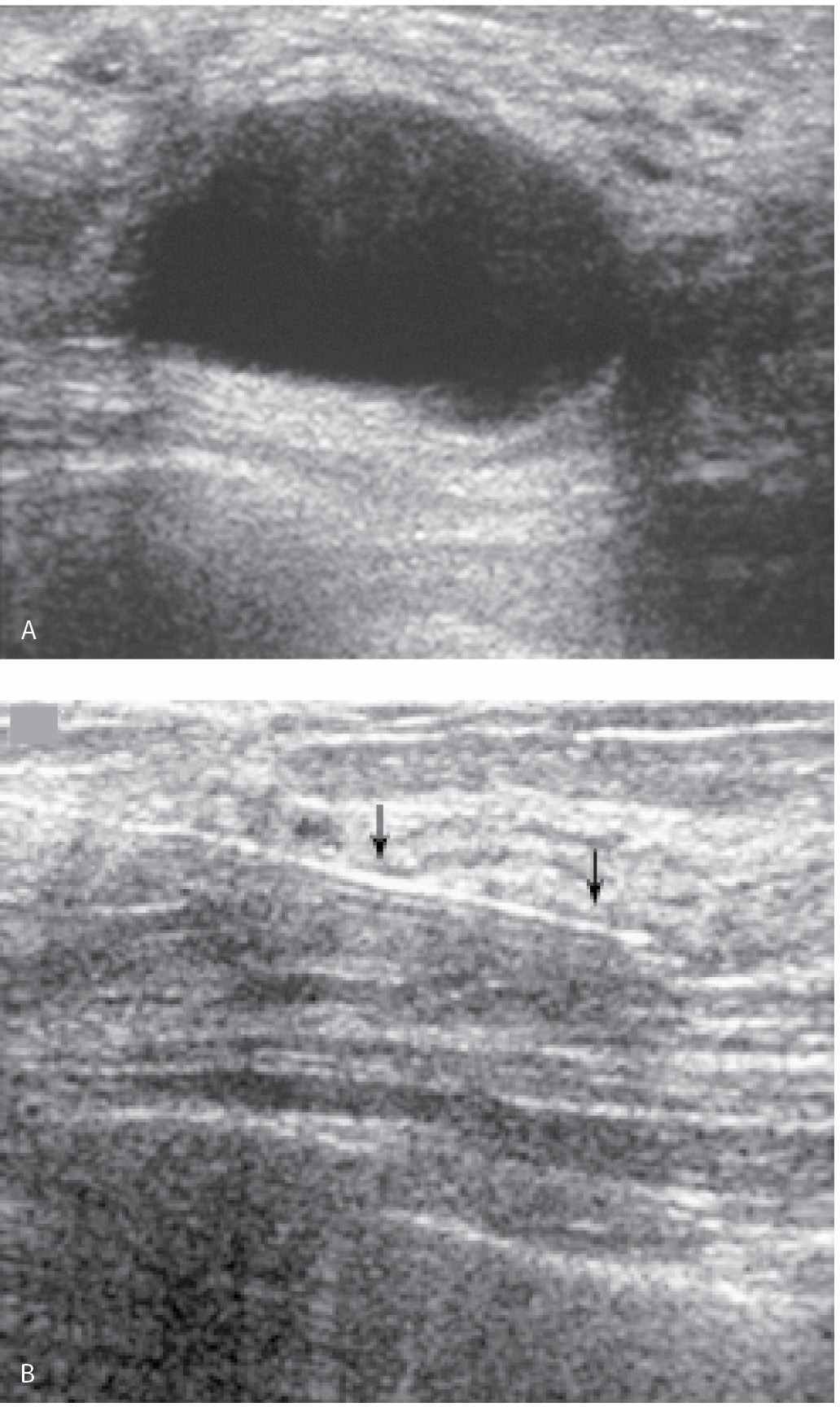
We consider aspiration when the ultrasound findings are not diagnostic of a cyst and sometimes do pneumocystography in patients in whom we suspect an intracystic or mural abnormality (Fig. 12.14). Palpation, ultrasound, or mammography can be used to guide the aspiration. Even with palpable cysts, our preference is to aspirate them using ultrasound guidance. Direct observation with ultrasound expedites needle movements and is helpful in determining the amount of pressure needed to puncture cyst walls that are sometimes thickened and inflamed. After the aspiration, no residual abnormality should be seen sonographically (Fig. 12.15). If a persistent abnormality is seen, a core biopsy is indicated. When pneumocystography is done, half the amount of aspirated fluid is replaced with air. Spot compression magnification views of the cyst are obtained in orthogonal projections (CC and 90-degree lateral views) to evaluate the cyst wall (Fig. 12.14C, F).
When using ultrasound guidance, we select an approach that enables us to introduce the needle parallel to the chest wall and transducer (see needle biopsy section for a more detailed description). The skin is cleaned with betadiene and alcohol. Lidocaine (1 or 2 mL of 1%) is used to slowly raise a skin wheal. Under ultrasound guidance, the anesthesia needle is advanced up to the cyst (but not into the cyst) and lidocaine is infiltrated along the expected course of the needle. A few minutes are allowed to elapse after the administration of the lidocaine. Using ultrasound guidance, a 20G spinal needle is used to puncture the cyst. When using real-time scanning, it is easy to appreciate how much pressure to apply in traversing the cyst wall. In some patients, the cyst wall indents significantly before it is pierced. With the tip of the needle in the center of the cyst (Fig. 12.14B), the inner stylet is removed and the needle is connected to a 10 mL syringe. Under direct ultrasound visualization, suction is applied until all of the contents are evacuated. There should be no residual abnormality following the aspiration. In patients with cysts having thick contents, an 18G spinal needle may be needed to completely evacuate the cyst. The aspirated fluid is not submitted for cytological evaluation unless the aspirate is bloody (or the patient requests it).
FIG. 12.15 • Cyst aspiration. A: Nearly anechoic cyst with posterior acoustic enhancement and internal echoes. The distribution of the echoes is such that they may not represent reverberation artifact. After local anesthesia is administered, a 20G needle is advanced into the lesion. An image to document needle positioning is done (not shown). B: The aspiration is monitored during real time and the echoes are seen being aspirated into the needle. No residual abnormality is seen post aspiration. This image done after the aspiration documents normal tissue surrounding the needle (arrows). No additional intervention or follow-up is indicated in this patient. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.).
Air can be injected into the cyst cavity. Some investigators have suggested that injecting air may have a therapeutic benefit by reducing the likelihood of cyst recurrence (2,8,9). In these patients, no mammographic images are obtained after the air is injected. Alternatively, a pneumocystogram (2,8,9) can be done for diagnostic purposes to evaluate potential intracystic or mural lesions. After the cyst is drained, the needle is stabilized and the fluid-filled syringe is replaced with a syringe containing air. Fifty percent of the aspirated fluid is replaced with air. The needle and syringe are then removed. Sonographically, an irregular echogenic line is seen if air is injected (Fig. 12.16). For a pneumocystogram, spot compression magnification views of the cyst are done to evaluate the wall of the cyst and the appearance of the surrounding tissue (Fig. 12.14).
In women with small lesions, or when the lesion is deep in a larger breast or deep in the tissue, cyst aspirations can be done using mammographic guidance (see preoperative wire localization section). A fenestrated paddle is used to establish the coordinates for the lesion. With the patient still in compression, a 20G spinal needle is advanced slowly at the coordinates for the lesion as suction is applied. When fluid is obtained, the needle is stabilized until all of the fluid is aspirated. The needle is left in place and follow-up orthogonal images are obtained to document resolution of the lesion.
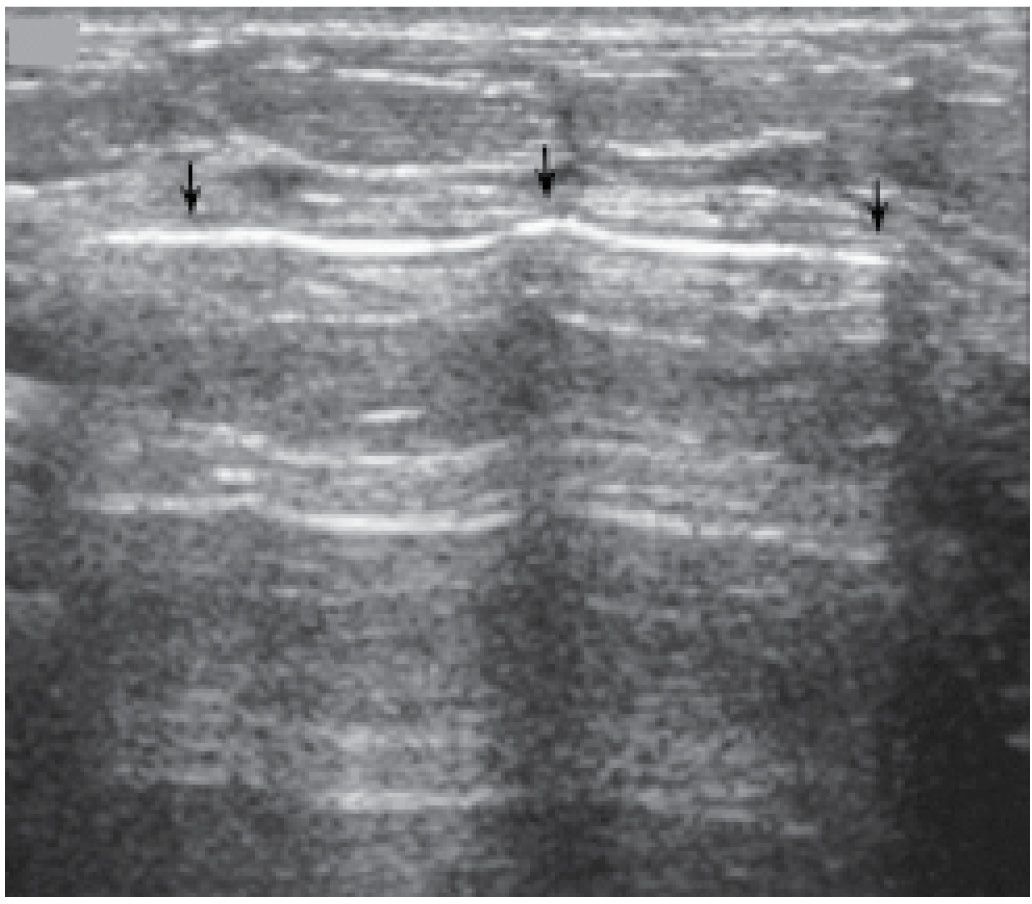
FIG. 12.16 • Air. An echogenic line (arrows) is seen following injection of air into a cyst cavity. Although not evident in this patient, shadowing may be seen in some patients deep to the injected air. In this patient, the air is injected in an attempt to reduce the likelihood of cyst recurrence. No mammographic images (e.g., pneumocystogram) are done post aspiration. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.).
Management of Patients with a Complex Cystic and Solid Mass
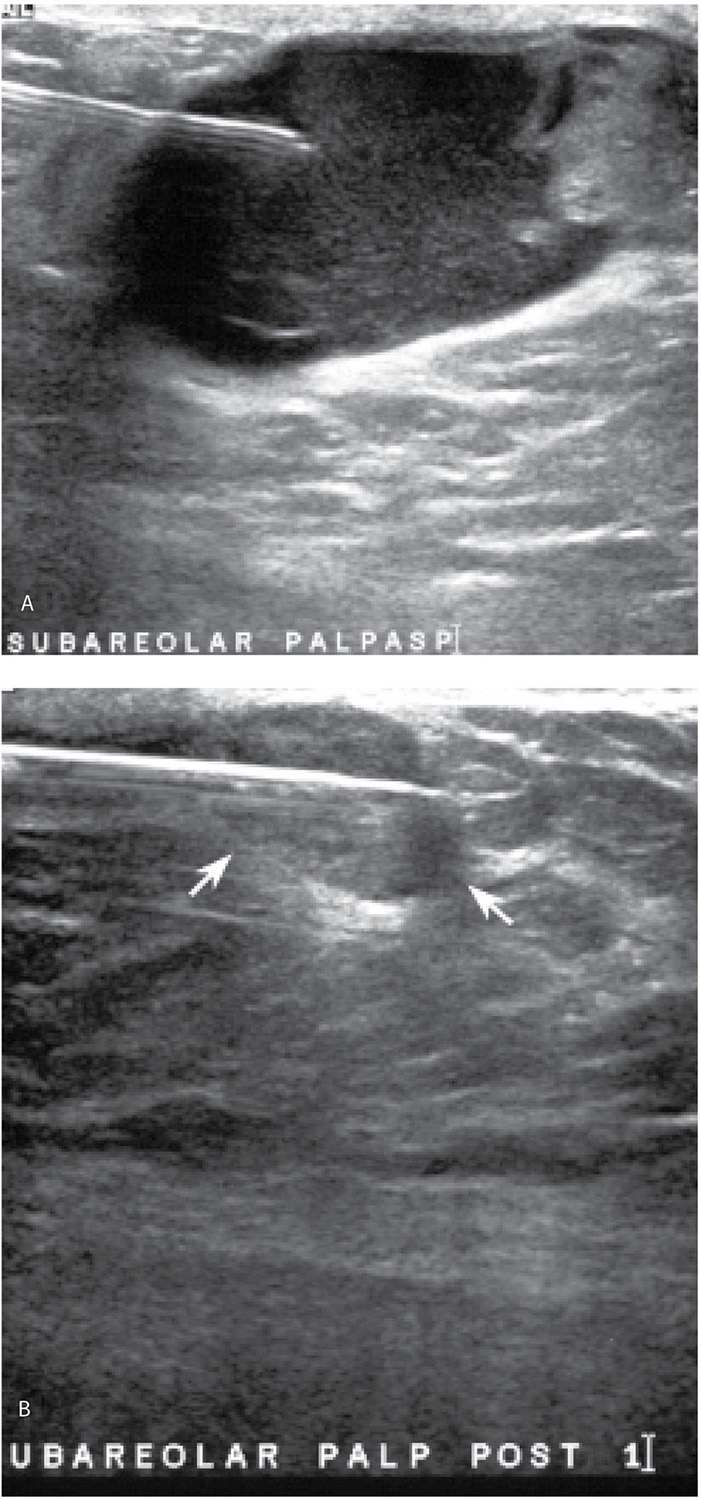
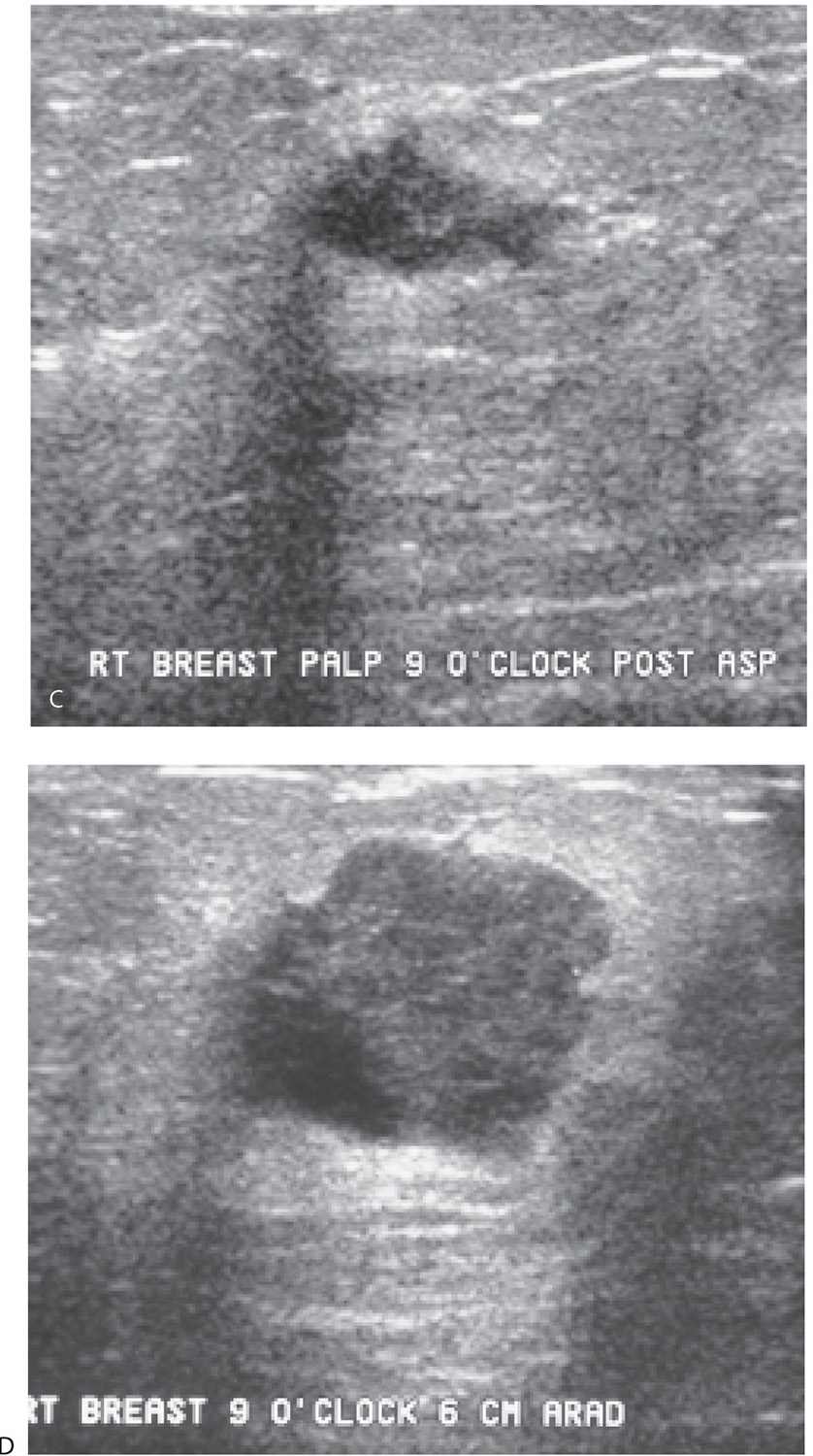
If a large fluid component is present in patients with a complex cystic and solid mass, our approach is to aspirate the fluid and do core biopsies through the residual solid component (Fig. 12.17). Even when malignancy is present, fluid cytology alone is nondiagnostic (or misleading) in approximately 50% of patients; the diagnosis is made on core biopsies of the solid component (Fig. 12.17). If fluid cytology is often nondiagnostic, why not just core the solid component without first aspirating the fluid out? The fluid in some of these lesions can incite a significant aseptic inflammatory reaction if fluid is inadvertently “spilled” into the surrounding tissue. When inadvertently induced, the inflammatory reaction simulates an acute mastitis with the patient describing significant tenderness, redness, and swelling of the breast. Since this is not bacterial, antibiotics are of no use. The patient is managed conservatively with symptoms subsiding slowly over several days to weeks. We try hard to avoid precipitating these and in our experience, concerns regarding an inability to identify the solid component or localize the lesion at a later date if the fluid is first aspirated are not well founded. If a tumor is present and the source of the fluid, it will be apparent after aspiration and in most patients, the fluid reaccumulates within several days of the aspiration (Fig. 12.18).
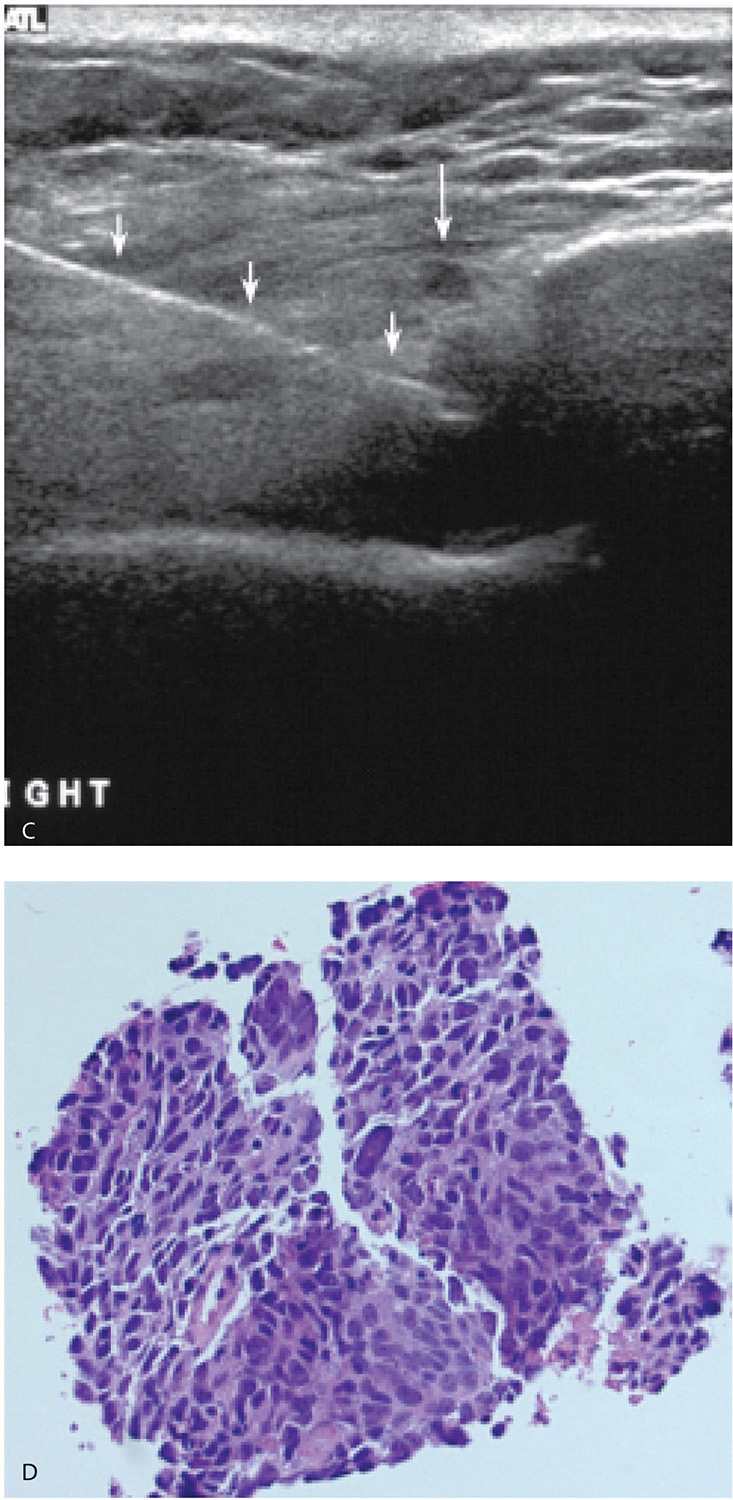
FIG. 12.17 • Encapsulated papillary carcinoma, intermediate to high grade. A: Complex cystic and solid mass in a 65-year-old man. A 20G spinal needle is used to aspirate the fluid. In this patient, three prior fine-needle aspirations done using palpation guidance yielded nonspecific benign results. A solid mass remains after the aspiration. B: Post fire image demonstrating the core needle through the residual solid mass (arrows). The diagnosis of a papillary carcinoma is made on the core biopsy and confirmed at the time of the mastectomy. Sentinel lymph node (LN) biopsy is negative (0/6 LNs). See Figure 10.14 for the diagnostic images on this patient.
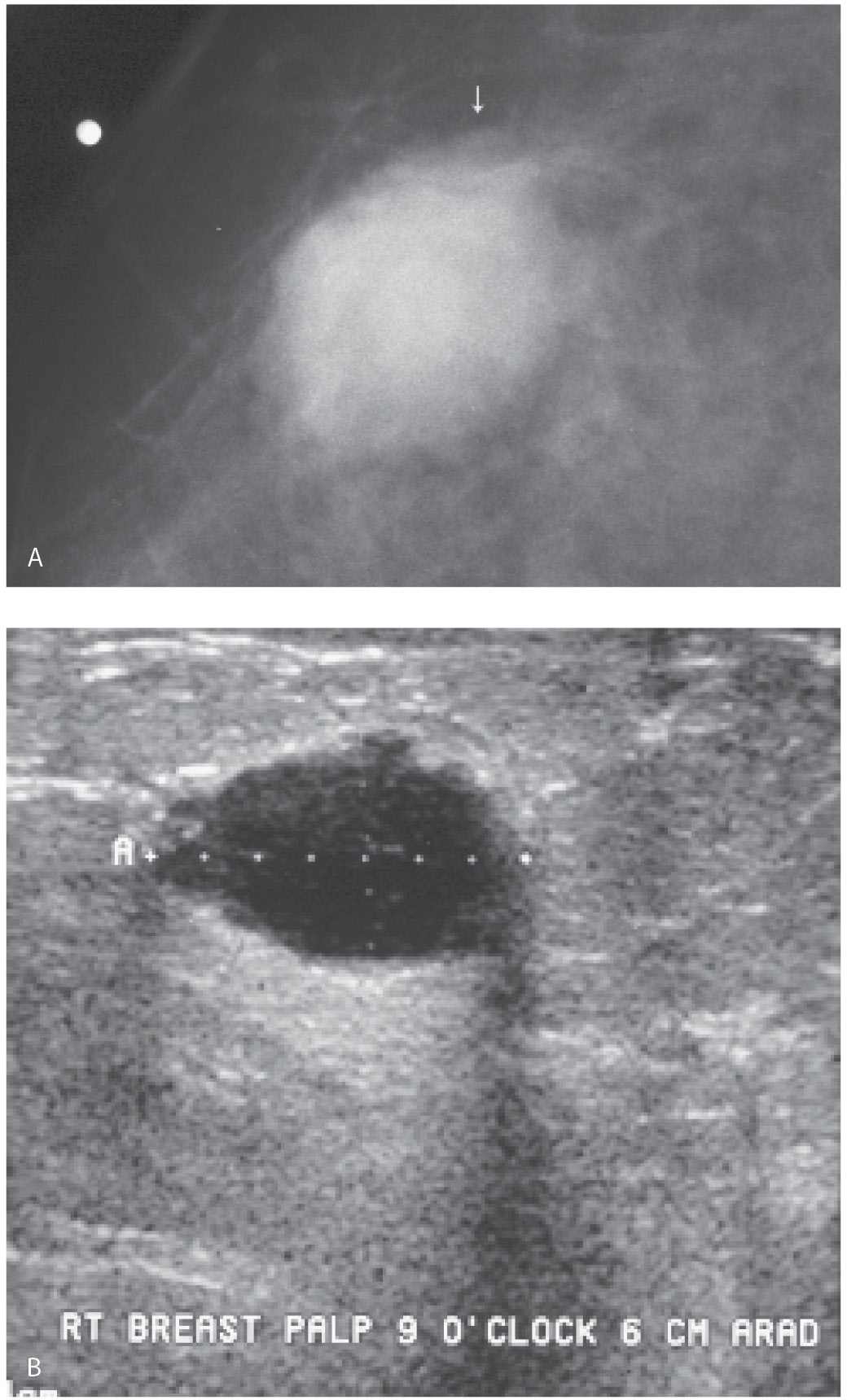
FIG. 12.18 • Invasive ductal carcinoma, not otherwise specified, poorly differentiated. A: Spot tangential view in a 36-year-old patient who presents with a tender, “lump.” Metallic BB marks the clinical finding. A round mass (arrow) with indistinct margins is imaged corresponding to the palpable finding. B: Ultrasound. A complex cystic and solid mass with lobulated margins and posterior acoustic enhancement is imaged correlating to the clinically and mammographically apparent mass. Closely evaluate the margins and note irregular microlobulations and spiculation. Aspiration is undertaken. C: Residual irregular abnormality is seen post aspiration; when abnormalities persist following aspiration, do a core biopsy. Fluid cytology is not diagnostic in approximately 50% of patients. In this patient, the fluid is submitted for cytology, and necrosis, inflammatory cells, and viable cells with atypia are reported. A course of antibiotics is prescribed and a follow-up ultrasound is scheduled. D: Ultrasound, 5 weeks after the aspiration. A round mass with a heterogeneous echotexture, angular margins, and posterior acoustic enhancement is imaged at the prior aspiration site. Ultrasound-guided core biopsy done to establish the diagnosis of an invasive ductal carcinoma, high grade. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
FINE-NEEDLE ASPIRATION
Fine-needle aspiration (FNA) is a quick, reliable method of accurately establishing a diagnosis for breast and axillary masses; it is used successfully and advocated by many (10,11). Others contend that inadequate specimens (reported in as many as 47% of patients) and false negative rates (sensitivity range of 65%–99%; specificity of 64%–100% and accuracy of 81%–98%) are unacceptably high (10,11). FNA can be successfully used in evaluating patients with palpable and mammographically detected masses; it is of limited usefulness in evaluating clusters of calcifications. As with anything, however, good technique and experience are invaluable in maximizing the usefulness of the technique. Also, having a trained cytopathologist who is comfortable with breast aspirates is critical; the pathologist needs to be comfortable in making definitive statements regarding the adequacy of the sample and the presence of malignancy. However, keep in mind that if you provide an adequate amount of cellular material, the diagnosis can usually be made. Ideally, the cytopathologist is available while the aspiration is done so that the adequacy of the specimen can be determined immediately. If additional material is needed, more samples can be obtained.
For nonpalpable lesions, fenestrated paddles, stereotactic devices, or ultrasound can be used to accurately localize the lesion. At this time, we are almost exclusively doing ultrasound-guided FNAs primarily for lesions that may otherwise be difficult to approach with a core needle biopsy (Fig. 12.19). In some patients, with breast and axillary findings, we obtain cores of the breast mass so that enough material is available for receptor analysis (estrogen and progesterone receptors and HER2/neu) and do an FNA of the axillary finding for confirmation of suspected metastatic disease. The predetermined skin entry point is wiped with betadiene and alcohol; lidocaine (1%) is used to raise a skin wheal and is infiltrated along the expected course of the needle. For one-handed aspirations, the needle (22G or 25G) can be attached to a syringe in a pistol grip holder. Alternatively, with the tip of the needle in the center of the lesion, the needle can be attached directly to a syringe. As suction is applied and, under ultrasound guidance, the needle is slowly and methodically moved from one end of the lesion to the other making sure the needle does not come out of the lesion (Fig. 12.20). As incursions are made, the angle of the needle can be varied. The suction is stopped before the needle is pulled out of the lesion. With the needle out of the breast, it is disconnected from the syringe and a syringe filled with air is attached and used to gently express the contents of the needle onto a glass slide. The material on the slide is smeared in one swift movement using another slide at a slight angle. Fixation is accomplished by allowing the material to air dry or placing the slide in 95% alcohol. In addition to the material smeared on slides for cytology, we also do one or two aspirates for cell block: the aspirated material is placed in normal saline and processed for cell block and hematoxylin and eosin staining (Fig. 12.19D). The methods used in handling the aspirates should be reviewed with the pathologist at your institution since there are variations.
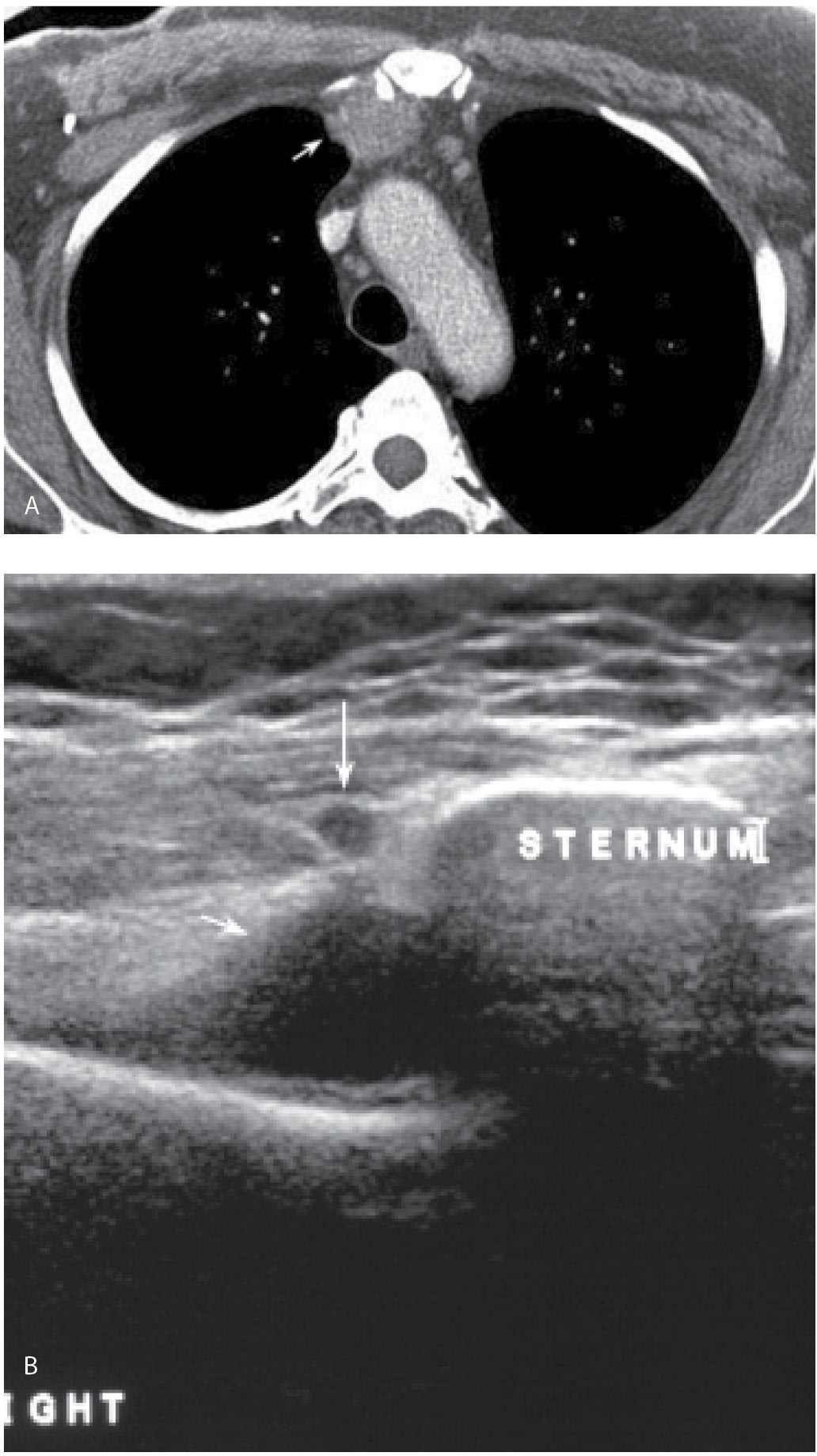
FIG. 12.19 • Fine-needle aspiration, internal mammary lymph node. A: Chest CT scan in a patient with a history of right breast cancer treated with mastectomy and axillary dissection. An abnormal internal mammary lymph node (arrow) is described. B: Ultrasound. A nearly anechoic mass (short arrow) is imaged adjacent to the sternum immediately deep to a pulsating vessel (long arrow). C: Under ultrasound guidance, a 20G spinal needle (short arrows) is advanced into the mass. The pulsating vessel (long arrow) is monitored during the aspiration. With the needle in the mass, suction is applied and slow methodical incursions and excursions of the needle in the mass are monitored during real time making sure the needle does not go out of the mass at either end. Material is submitted on a slide for cytology and aspirates are also submitted in normal saline for cell block (hematoxylin & eosin, histology). D: Cell block material demonstrating malignant cells consistent with metastatic breast cancer. Notice the amount of cellular material available following fine-needle aspiration with the technique described.
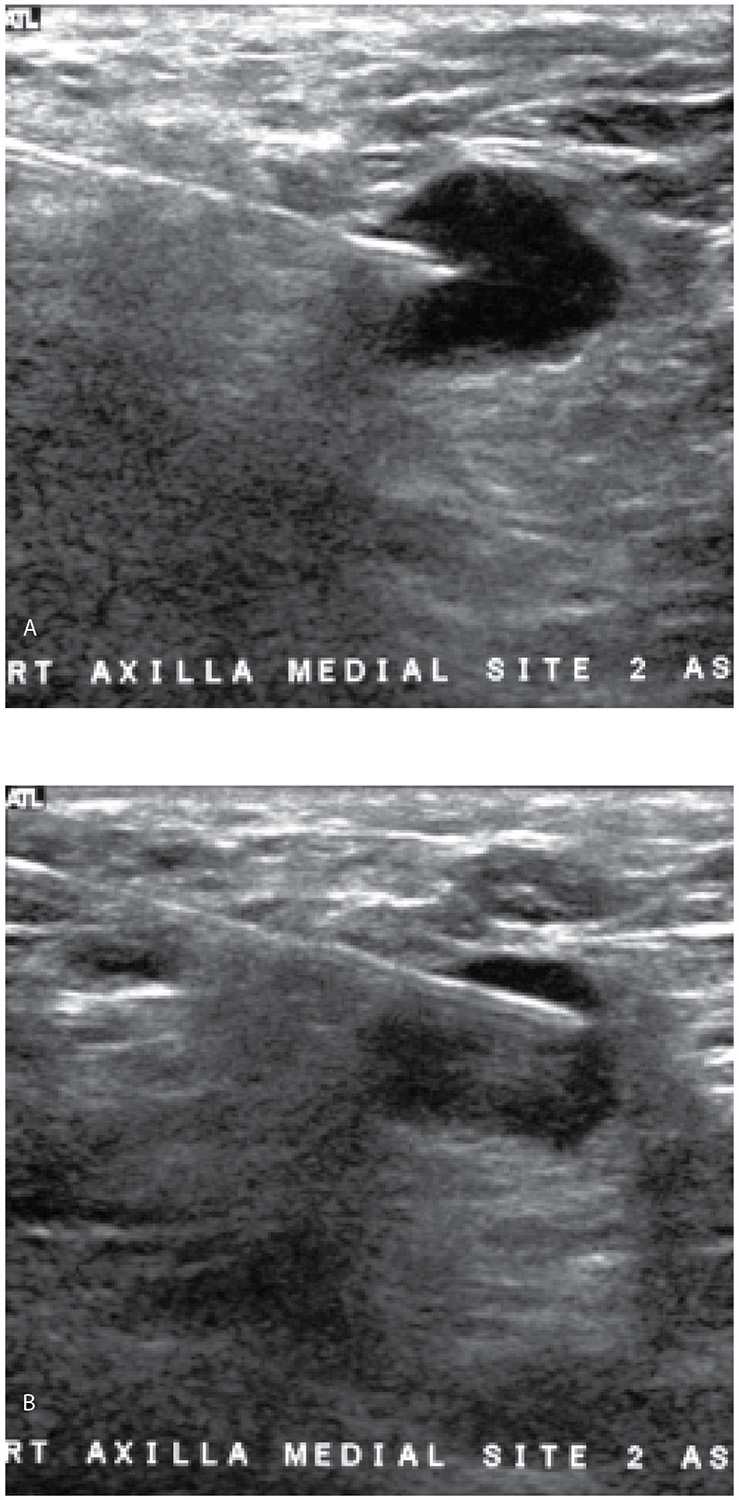
FIG. 12.20 • Fine-needle aspiration, axillary lymph node. A: Anechoic mass with some posterior acoustic enhancement in the right axilla likely representing metastatic disease to a lymph node in a patient with ipsilateral breast cancer (mammogram and ultrasound of breast mass not shown). Under ultrasound guidance, a 20G spinal needle is advanced to the superficial portion of the mass. Suction is applied. B: With suction being applied and under ultrasound guidance, the needle is slowly advanced through the mass but not beyond it. These two images illustrate the slow, repeated movements of the needle as suction is applied. With the needle tip just in the mass as shown in part A, suction is applied. Slow, methodical incursions and excursions of the needle from one end of the mass to the other are monitored with ultrasound. All of the movements of the needle when suction is being applied occur in the mass. After three or four movements (A to B to A, etc.) in the mass, suction is stopped and the needle is pulled out. Aspirates are submitted for cytology and histology (e.g., cell block). Metastatic breast cancer is reported with associated lymphoid elements.
At the onset of this discussion, it is important to point out that needles, guns, needle vacuum systems, and clips proliferate almost on a monthly basis. It is not my intention to discuss all available options exhaustively or even the pros and cons of each but rather to suggest that the tool selected is not nearly as important as the needed observations, evaluations, generation of appropriate differentials, and the critical thinking required to take care of patients. It is easy to biopsy every potential abnormality (including those that are obviously benign) with the largest and latest gadget, after which one or multiple clips are left behind. But is this really what is needed for optimal patient care? Why not approach each patient individually and, based on suspected histology (e.g., if invasive lobular is suspected, FNA of an axillary lymph node may not be the best tool to assess for metastatic disease), use the simplest approach that will provide an accurate diagnosis? In establishing the extent of disease, do we need to biopsy four or five sites or can we biopsy the extremes of a lesion or the two lesions that are furthest apart? Is vacuum assistance needed for every breast biopsy that is done? If vacuum is used, how many cores are needed: 5, 10, 20, or 50 and should the number of cores done be the same for all patients? If one or two cores with a 14G needle provide an accurate answer, why is this not done more often?
Some adamantly insist that clips need to be left in all patients after imaging-guided biopsies. Really? Is a clip really indicated in all patients following imaging-guided biopsies? Why not consider what is appropriate for the patient you are taking care of and tailor your approach accordingly? If the patient wants a mastectomy if diagnosed with breast cancer, why is a clip needed? If a patient with a 2- to 3-cm mass or one with segmentally distributed malignant-type calcifications wants a lumpectomy, are we suggesting that the percutaneous biopsy will leave no residual abnormality for the localization? For those who argue that the clip is useful in establishing concordance between a biopsied mass on ultrasound and the mammographic finding, I submit to you that this type of concordance needs to occur before any biopsy is done. There are simpler ways to establish mammographic–sonographic concordance than doing a vacuum-assisted biopsy, leaving a clip, and then finding out that you have not biopsied the area in question mammographically. Doing things because we can or haphazardly is not the answer. So, in this section I provide an overview of the basic principles and will discuss our approach and application of the basics. We strive to do what is needed to manage our patients appropriately. Optimal patient care is our compass, and critical, clinically-oriented thinking regarding what is best for our patients guides our approach. Accurate answers can often be obtained simply and expeditiously.
First described by Parker and associates, stereotactic-guided, and then ultrasound-guided, biopsies of breast lesions represent a significant advancement in patient care and management. These authors first described the use of a spring-loaded mechanism with a 14G needle and then a vacuum-assisted device that can be used with 14G, 12G, 11G, and 8G needles to increase the amount of tissue obtained (12–16). These techniques revolutionized the practice of breast imaging and are now well established with significant literature to support their routine use (12–22).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree