Abstract
Intraabdominal masses are rare and often only recognized in the third trimester of pregnancy. There are typically multiple options for diagnosis, but the differential diagnosis can be narrowed by considering the locality and consistency of the lesion. Magnetic resonance imaging may be useful. Some cases will have other concurrent pathology. Karyotyping may be needed.
Keywords
intraabdominal mass, intestinal diseases, cysts, diagnostic imaging
Introduction
Intraabdominal solid masses and cystic lesions are not commonly identified during the routine 20-week anomaly ultrasound (US) scan and are uncommon findings at later gestations. However, intraabdominal masses occasionally are seen as an incidental finding during a third-trimester US scan assessing fetal growth and wellbeing. Potential differential diagnoses are numerous, but the differential diagnosis typically can be restricted through a detailed assessment of the site and appearance of the lesion. Prenatal intervention is not indicated in most circumstances, but further postnatal investigation or surgical repair may be required shortly after birth, so effective prenatal diagnosis avoids delay in treating the neonate.
Disease
Definition
An intraabdominal mass is a space-occupying lesion within the abdominal cavity that appears either cystic or solid.
Prevalence and Epidemiology
Intraabdominal masses are rare. The prevalence of congenital anomalies, such as bowel atresias, is low. Duodenal, small and large bowel, and rectoanal atresias affect 5.1 : 10,000 births, accounting for 2.2% of congenital anomalies. The prevalence of atresia of the common bile duct is even lower (0.3 : 10,000 births).
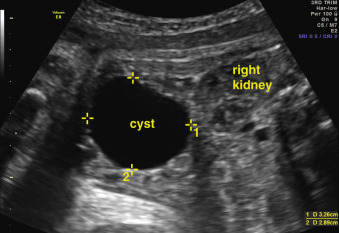
Some lesions, such as ovarian cyst or hydrometrocolpos, affect only female fetuses. A significant ovarian cyst is subjectively defined as greater than 2 cm in diameter and occurs approximately in 4 : 10,000 female live births. Choledochal cysts are more common in females (4 : 1 prevalence) and in Asian populations. Other anomalies, such as enteric duplication cysts, are more common in males than in females (2 : 1). However, because of the low birth prevalence, it is often difficult to determine accurate epidemiologic differences between populations. Abdominal cysts are discussed in more detail in Chapter 24 .
The most common echogenic intraabdominal mass is echogenic bowel ( Chapter 22 ). In contrast to other intraabdominal masses, this acquired anomaly may be seen at the 20-week scan and has a prevalence of 0.3% at this gestation. Greater than 80% of fetuses with echogenic bowel have no underlying pathology, but there are associations with chromosomal abnormality, cystic fibrosis, fetal infection, and intrauterine growth restriction.
Etiology and Pathophysiology
The etiology of intraabdominal masses varies widely depending on the organ or system involved. Numerous abnormalities, in particular, cystic masses, are truly congenital with failure in the development of hollow viscera, such as a portion of the bowel (e.g., duodenum, small or large bowel, rectum-anus; Chapter 26 ), or of drainage channels (e.g., common bile duct; Chapter 25 ). Most of these anomalies, with the exception of duodenal atresia, are not associated with chromosomal abnormality.
Small bowel atresia is thought to result from an episode of vascular impairment during embryologic development. It may occur in isolation or in association with gastroschisis (the cause of which is also considered to be a vascular accident), or a volvulus. In contrast, enteric duplication cysts appear to arise from a failure of separation between the notochord and endoderm, which explains an association with vertebral anomalies. Some congenital anomalies, such as cystic fibrosis, cause secondary changes that are visible as an intraabdominal mass. The increased echogenicity of the bowel is acquired owing to thick, abnormally viscous meconium, and the development of a pseudoobstruction.
Some intraabdominal masses are acquired rather than congenital. The inflammatory response to fetal infection can lead to the development of echogenic bowel and foci of calcification. The obstructed or hypoxic small bowel may become distended, and perforation may lead to a sterile chemical peritonitis with calcified foci being seeded through the peritoneal cavity as part of the inflammatory response. These foci represent a subacute sign of injury that may take a few weeks to evolve (see Chapters 22 and 26 ).
Focal calcification is also occasionally seen within the liver, with a prevalence of approximately 6 : 10,000 fetuses (see Chapters 23 and 28 ). These calcifications may result from localized vascular disruption within the liver causing infarction or ischemia and subsequent fibrosis and calcification. Calcifications are generally found in three sites: on the surface of the liver, where they may be associated with meconium peritonitis; as discrete lesions within the liver capsule, associated with vascular emboli; or more diffusely throughout the liver parenchyma as a result of more widespread ischemia and necrosis that may occur secondary to fetal infection.
Ovarian folliculogenesis occurs from 20 weeks’ gestation, and fetal ovarian cysts are often not recognized until the third trimester. The hypothalamic-pituitary-ovarian endocrine axis is active from the end of the first trimester, and cyst formation is thought to be related to gonadotropin, placental human chorionic gonadotropin, or placental estrogen release.
Tumors are rarely seen in the fetus, but hemangioma (60%), mesenchymal hamartoma (23%), and hepatoblastoma (17%) are the most common among perinatal hepatic tumors (see Chapter 28 ). Neuroblastoma and teratoma have also been reported, but are rare, except for sacrococcygeal teratomas that often have a pelvic component ( Chapter 31 ). Hepatic tumors may manifest as a solitary or multifocal hypoechoic or hyperechoic mass within the liver and may have associated areas of calcification. Hepatoblastoma is associated with increased alpha-fetoprotein, and this may be reflected in maternal serum alpha-fetoprotein levels.
Manifestations of Disease
Clinical Presentation
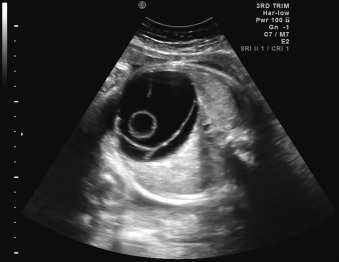
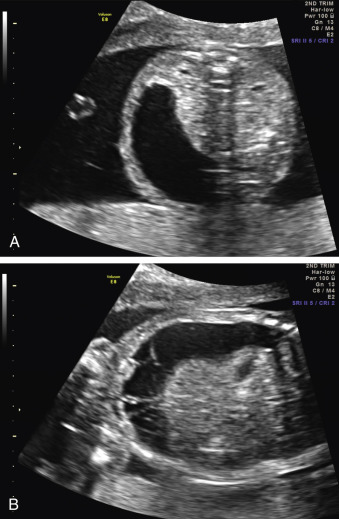
Because the gastrointestinal (GI) tract does not reach functional maturity until the third trimester, pathology is often not recognizable before this stage. The fetus swallows two to seven mL of amniotic fluid every 24 hours at 16 to 17 weeks’ gestation, and this increases to 13 to 16 mL at 20 weeks, and 450 mL at term. Fetal bowel motility also increases with gestational age. Consequently, anomalies such as duodenal atresia can be identified when the proximal duodenum fills with amniotic fluid and becomes distended, developing the characteristic double bubble sign in the late second or early third trimester of pregnancy ( Fig. 29.1 ). Generally, the lower an obstruction is in the GI tract, the later and more subtle its presentation. Anomalies such as Hirschsprung disease and anal atresia are rarely diagnosed before birth. Similarly, pathologies such as ovarian cysts become visible only in the third trimester of pregnancy, owing to the normal developmental process ( Fig. 29.2 ).
Imaging Technique and Findings
Ultrasound.
A fetal morphology scan is typically performed at 18 to 23 weeks’ gestation and includes standard views of the abdomen and abdominal contents ( Chapter 1 ). This examination is designed to detect mainly more common, major structural anomalies, such as omphalocele, gastroschisis, diaphragmatic hernia, and significant urogenital abnormalities. The abdominal circumference is measured in an axial section at a level where the stomach and intrahepatic portion of the umbilical vein can be seen. Careful examination of this and adjacent sections, reveals most of the anomalies mentioned when they are large enough for detection on US. The bowel should be visualized to check for evidence of echogenicity (being as bright as bone) or dilatation (which should be formally measured in transverse diameter).
At more advanced gestational ages, when fetal growth is assessed, the biometry of the abdomen is measured using the same previously described landmarks. Although morphology is not systematically reviewed in the third trimester, if it has been assessed at the second-trimester scan, the sonographer should be aware of the potential for intraabdominal pathology to become apparent at later gestations, and should note the presence of any cystic or solid masses while scanning through the abdomen to assess biometry.
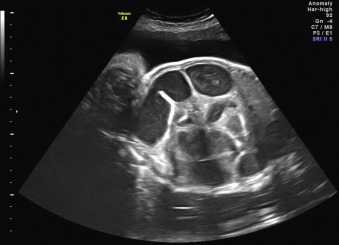
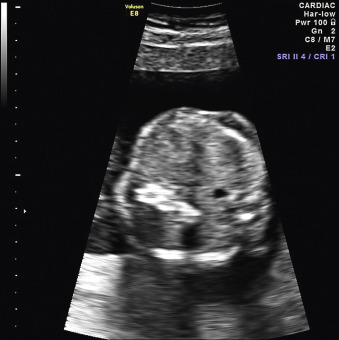
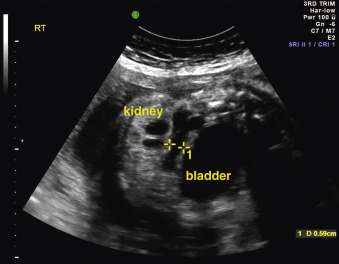
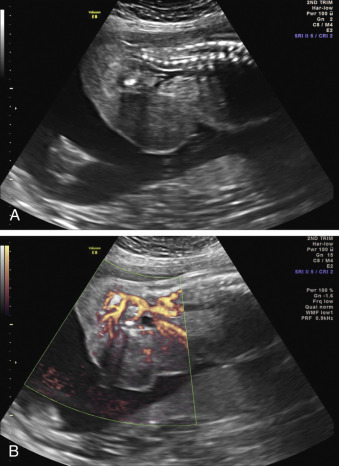
When a cystic or solid mass is shown, it is important to extend the examination to characterize it in more detail. By changing the insonation angle (i.e., by rotating the US probe around the maternal abdomen), it is normally possible to image the mass from different angles, which may help to delineate its border and define whether it is contained within a single viscus, or is more diffuse. Similarly, visualization in different fetal planes (axial/coronal and sagittal) allows localization ( Figs. 29.3 and 29.4 ). Adapting the settings of the US system (gain, contrast, gray mapping, speckle reduction imaging, tissue harmonics, real-time compounding) may help to define the border of the mass more clearly, and allow better contrast between adjacent soft tissue structures. Cystic lesions may be simple or complex, and solid lesions may be homogeneous or heterogeneous. Areas of calcification may be identified by loss of acoustic signal behind the mass (shadowing). Color or power Doppler can be used to identify a vascular pedicle or more widespread perfusion ( Fig. 29.5 ).
Certain anomalies have defining characteristics: objective assessment of the bowel relies on normative data produced by two studies involving 430 fetuses. Dilatation of the small or large bowel is defined by a maximal luminal diameter greater than 7 mm for the small bowel, and greater than 18 mm for the large bowel ( Fig. 29.6 ). Similarly, an ovarian cyst is considered relevant if it is more than 2 cm in diameter ( Fig. 29.7 ) and may have simple septations or daughter cysts, which are considered to be pathognomonic. Whilst identification of an abdominal or pelvic cystic mass is the most common feature of a cloacal abnormality, it is often associated with other intraabdominal pathologies including hydronephrosis and hydroureter, oligohydramnios, distended bowel, and ascites. Calcified foci are defined as discrete areas of echogenicity with acoustic shadowing. These may be free within the peritoneum, within the stomach or bowel wall, or within the liver ( Fig. 29.8 ) or spleen, or associated with the biliary tree.