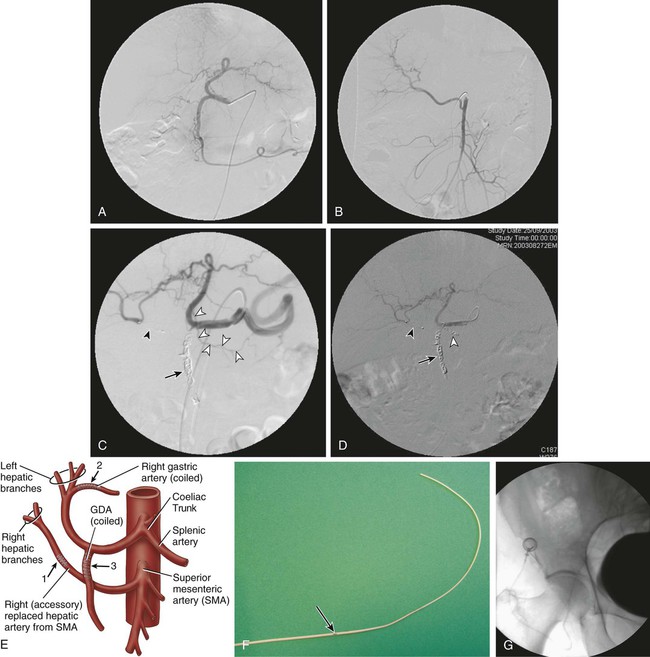
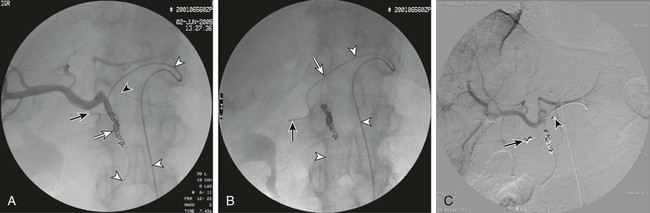
Because intraarterial hepatic chemotherapy (IAHC) is a local treatment, it is used in cases of liver metastases without or with very limited extrahepatic disease. It is often used as a rescue after failure of intravenous (IV) therapies for metastases, and provides a high response rate even using drugs that were or became inefficient with IV administration. Because it is highly efficient, IAHC might also be used as inductive therapy in chemo-naive patients with nearly resectable liver metastases. In such settings, the goal is to obtain the highest tumor response possible as early as possible in the disease to downstage a nonsurgical candidate to a surgical candidate. Indeed, it has been demonstrated that the increase in response rate of colorectal liver metastases (CRLM) to treatment is linearly correlated with an increase in resection rate, and consequently with an increased chance of cure.1 • 5F catheter and guidewire for visceral angiography • To avoid blood leak after indwelling catheter insertion, introducer sheath must not be used. • Microcatheter (2.4F) plus 0.016-inch guidewire • Microcoils (0.018 inch; 2/5 mm to 4/8 mm in diameter) • Exchange stiff 0.018-inch guidewire (Muso [Terumo Medical Corp., Somerset, N.J.] or V18 [Boston Scientific, Natick, Mass.]) • Indwelling catheter tapered from 5F to 2.7F, with a side hole (ST-305C, [B. Braun Medical, Center Valley, Pa.]). The kit has a subcutaneous port. Until recently, the unique route of catheter insertion was by laparotomy with retrograde cannulation of the gastroduodenal artery (GDA). The most often described access route for percutaneous technique is the axillary or femoral artery, with a port positioned on the chest wall or lower abdominal wall.2–4 We prefer the femoral route for implantation because this access is always necessary for flow remodeling, which is always needed as a first step before indwelling catheter insertion. Flow remodeling is needed to infuse chemotherapy in the complete liver, and only in the liver, through a single artery. Single artery means that replaced hepatic arteries, if present, must be occluded proximally with stainless steel coils to maintain a patent single artery for indwelling catheter insertion (Fig. 74-1, A-C). In the case of replaced hepatic arteries, the hepatic artery that will be kept patent and selected for indwelling catheter insertion will be the one that bears the GDA or the largest one (Fig. 74-1, D and E). Infusion of drug only in the liver means that every single artery arising from the hepatic artery downstream of the infusion hole in the catheter must be occluded to avoid infusion to neighboring organs, including the stomach, duodenum or pancreas. In clinical practice, the GDA and right gastric artery (RGA) are the arteries that nearly always require an endovascular occlusion because it is rarely possible to place the perfusion hole of the catheter downstream of them (see Fig 74-1, D). The absence of reported toxic effects on the gallbladder make it unnecessary to systematically occlude its feeding vessels, but a very large cystic artery should probably be occluded. Indeed, even though cholecystectomy has been described as a necessary step in preventing cholecystitis secondary to drug diffusion to the gallbladder through the cystic artery at the time of surgical catheter placement, it is worth emphasizing that no cholecystitis has been reported in four series that studied over 250 patients with percutaneously implanted port catheters, including only 11 cholecystectomized patients.2,4–6 Because of the high risk of tip migration, the IAHC catheter must not be placed floating in the hepatic artery lumen. The indwelling catheter has to be inserted deeply into the GDA to provide stability of tip location. This indwelling catheter has a side hole that allows drug delivery (Fig. 74-1, F); it is placed in the common hepatic artery upstream of the arising of the GDA.6 The side hole is located 20 cm from the tip when the catheter is provided, and usually the catheter is shortened to have the side hole 7 to 8 cm from the tip as a compromise between pushability over the wire at time of insertion and stability after placement of the indwelling catheter. In practice, after the initial angiogram and occlusion of the replaced hepatic artery (if present) and RGA, the GDA is catheterized as distally as possible down to the distal right epiploic artery with a microcatheter. The microcatheter is fed with a 0.018-inch stiff exchange wire that will be used to insert the indwelling catheter. To increase pushability of the indwelling catheter over the 0.018-inch exchange wire, the microcatheter can be placed inside the indwelling catheter over the wire. The GDA will then be occluded around the indwelling catheter, using the side hole of the indwelling catheter to insert a microcatheter that will allow occlusion of the GDA with 0.018-inch coils. Although the use of cyanoacrylate glue has been reported for this purpose, in our own experience, we never use glue because we have found coils much safer than liquid embolic in the GDA. The goal of coil/glue occlusion of the proximal GDA is to provide both fixation of the catheter and occlusion of the GDA (see Fig. 74-1, D and E). When the GDA cannot be catheterized, does not exist, or has been ligated, the tip of the indwelling catheter can be placed in a peripheral branch of the hepatic artery, and the side hole is left in the hepatic artery. After angiographic control of the correct location of the catheter, providing complete and unique liver perfusion (Fig. 74-2), the catheter is tunnelized and linked to a port placed either on the chest wall or the pelvic wall, according to access route. Catheter maintenance means flushing with heparin solution (5000 units/10 mL) after completion of chemotherapy until the next course. Using the femoral artery for port catheter insertion is technically more challenging but can be achieved nowadays in the vast majority of patients thanks to improvements in catheter and materials designs. Femoral access will most often be needed for endovascular flow remodeling, even if the indwelling catheter is inserted through the axillary route. Since the first report of percutaneous port implantation for IAHC by Arai et al.7 in 1982, the axillary route has been much more used than the femoral route. It is preferred because it allows easier insertion of the catheter into the hepatic artery, which usually has a descending orientation in the initial part of the celiac trunk. One thus avoids the sharp angulation encountered when using femoral access. The main disadvantage of the axillary route is a higher rate of overall and severe complications, including up to 3% of aneurysms requiring arterial stents for treatment. It may also induce axillary artery thrombosis4 and a 0.5% to 1% rate of stroke.2,3,8 Aneurysms are caused by the difficulty of accessing and manually compressing the axillary artery. This leads some teams to access the axillary artery by surgical exposure and cutdown of a small branch, such as the thoracoacromial artery.3 Strokes occur when the body of the catheter lies in front of the origin of the left vertebral artery. For some authors, the retrieval or exchange of such catheters is risky enough to make them recommend that such a maneuver be performed through a femoral access if possible.3 The technical success rate of catheter insertions is very high in our experience and close to 100% in most series, including the largest recently reported one.3,9 Even if it is more time consuming, the catheter-blocking technique must be preferred to free-floating catheters; the risk of tip dislodgement is reported to be over 20% with a free-floating catheter in the hepatic artery.10 The largest series of percutaneous catheter implantations reported patency rates of 91%, 81%, and 58%, respectively, at 6 months, 1 year, and 2 years, allowing 3 to 102 courses of chemotherapy (mean = 35).3 A study comparing percutaneously and surgically placed port catheters reported the same rate of complications, with significantly shorter hospital stay and lower analgesic requirements for the percutaneous group (1.8 ± 0.7 days and 2 ± 0.9 doses) versus the surgical group (8.2 ± 22 days and 9.7 ± 3.2 doses).5 We recently compared percutaneous and surgical insertion of port catheters for IAHC and demonstrated that the success rates of implantation were 97% (65/67) for percutaneous placement and 98% (58/59) for surgical implantation.6 Among 107 patients, primary functionality was not different for percutaneous placement (n = 4.80 courses) versus surgical implantation (n = 4.82 courses), but functionality after revision was significantly higher for percutaneous versus surgical placement (9.18 vs. 5.95 courses, P = 0.004). This increased secondary functionality is due to easier revision of percutaneously placed ports versus surgical ones. The rates of discontinuation of IAHC linked to complications of the port catheters were 21% for percutaneous and 34% for surgical implantation.6 Overall, the rate of reintervention to maintain patency of the port catheter system varies from 20% to 30% according to reports. The reinterventions are most often due to gastric pain and the need for embolization of a previously nonoccluded RGA or occlusion of a collateral vessel to an occluded right gastric. The main complication of IAHC through percutaneous implanted ports is gastric ulceration occurring in patients in whom the RGA could not be occluded. We reported that intraarterial oxaliplatin and systemic 5-FU resulted in an objective response rate of 64% in colorectal cancer metastases that developed in chemo-naive patients, and 45% in patients resistant to IV oxaliplatin or irinotecan.11,12 The dramatic incidence and amplitude of response after intraarterial oxaliplatin led us to operate on 20% of initially unresectable patients by combining radiofrequency ablation and hepatectomy.12 Nevertheless, IAHC greatly affects liver function, reflected by impaired indocyanine green clearance, with rates up to 60% at 15 minutes. Interrupting IAHC allows a progressive normalization of this clearance within 2 to 4 months. Additionally, the macroscopic and microscopic aspects of the liver parenchyma are greatly modified, with infraclinical sclerosing cholangitis, central vein fibrosis, and moderate hepatocellular necrosis or cholestasis in the centrolobular area.13 More recent IAHC regimens have combined IV chemotherapy and even targeted therapies to provide very impressive results. Recently, 49 patients with unresectable CRLM (53% previously treated with chemotherapy) received IAH floxuridine and dexamethasone plus systemic chemotherapy with oxaliplatin and irinotecan.14 In this study, more than five CRLMs were present in 73% of patients; 98% had bilobar disease, and 86% had six or more segments involved. Of the 49 patients, 92% had complete (8%) or partial (84%) response, and 47% (23/49) were able to undergo resection in a group of patients with extensive disease. For chemotherapy-naive and previously treated patients, the median survival from the start of IAH therapy was 50.8 and 35 months, respectively. In our center, we treated 36 patients with extensive nonresectable CRLM (≥4 LM in 86%; bilobar LM in 91%) using IAHC with oxaliplatin (100 mg/m2 in 2 hours) plus IV 5-FU–leucovorin (leucovorin, 400 mg/m2 in 2 hours; FU, 400 mg/m2 bolus then 2400 mg/m2 in 46 hours), and cetuximab (400 mg/m2 then 250 mg/m2/wk, or 500 mg/m2 every 2 weeks) as first-line treatment.15 Overall response rate was 90% (95% confidence interval [CI], 70-99), disease control rate was 100% (95% CI, 84-100), and 48% of patients were downstaged enough to undergo R0 resection and/or radiofrequency ablation. After a median follow-up of 11 months, median progression-free survival (PFS) was 20 months (median overall survival [OS] not reached; 12- and 18-month OS, 100%). Catheter tip dislodgement is reported in more than 20% of catheters that have been left free-floating in the hepatic artery,10 and such technique must be avoided. Thrombosis of the hepatic artery is rare and seems to be related to the size of the indwelling catheter (e.g., when catheters > 5F are placed in the hepatic artery). Some authors have reported that free-floating catheters are more prone to induce thrombosis due to the moving tip, which can damage the arterial wall. The most frequent and severe complications of IAHC are related to extrahepatic perfusion of the anticancer drug, including gastric ulceration occurring in patients in whom the RGA could not be occluded. Indeed, a rate of 36% of acute gastric mucosal lesions has been reported in patients undergoing IAHC without embolization of the RGA, but this rate decreased to 3% in patients with an embolized right gastric artery.16 We have had the same experience without and with RGA embolization—30% and 5% (P
Intraarterial Ports for Chemotherapy
Regional Chemotherapy for Liver Tumors
Indications
Equipment
Standard Implantation
Technique
Controversies
Outcomes
Complications
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Intraarterial Ports for Chemotherapy