Fig. 8.1
Illustration of the different types of IPMNs. (a) Side-branch duct-type. (b) Multifocal branch duct-type. (c) Main pancreatic duct-type. (d) Combined-type
IPMNs are classified according to their origin
Branch duct-type IPMN
BD-type IPMN
Multifocal branch duct-type IPMN
MBD-type IPMN
Main duct-type IPMN
MD-type IPMN
Combined-type IPMN
C-type IPMN
Branch duct (BD)-type IPMN
Occurs in younger patients
More common in the uncinate process
May also involve the body and tail of the pancreas
Up to 41 % multifocal (MBD-type IPMN)
Most likely to harbor benign lesions (80.5 %)
Lower risk of malignant transformation when compared to MD-type IPMN
Main pancreatic duct (MD-type IPMN) (segmental or diffuse)
The majority arise within the head of the pancreas and progress distally with or without involvement of the side branches.
Most likely to harbor malignant lesions.
Combined-type IPMN (C-type IPMN)
Mixed main and branch duct-type
8.4 Histopathology
Cell of origin
Epithelial cells of the pancreatic duct
8.4.1 Macroscopic Characteristics (Figs. 8.2–8.5)
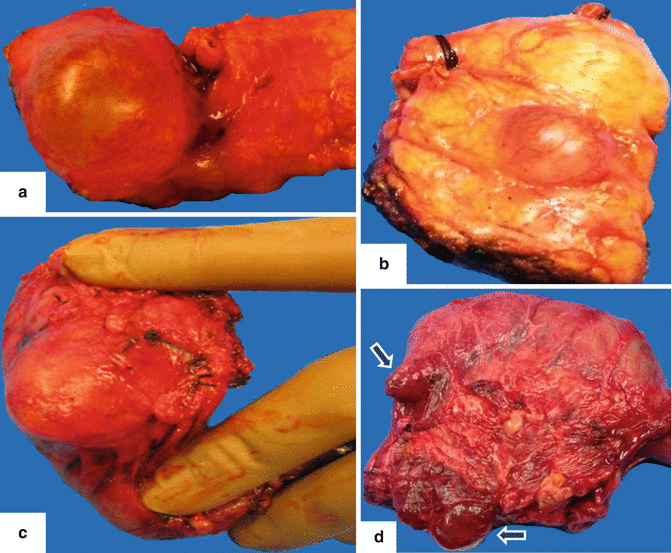
Fig. 8.2
IPMN gross appearance. Photographs of four different resected specimens of side-branch IPMNs show the wide variation in appearance of these cystic tumors. a: large, unilocular cystic mass. b: small, unilocular cystic mass. c: ovoid, well-defined cystic mass. d: two small, lobulated cystic masses in the pancreatic head adjacent to the duodenum (arrows)
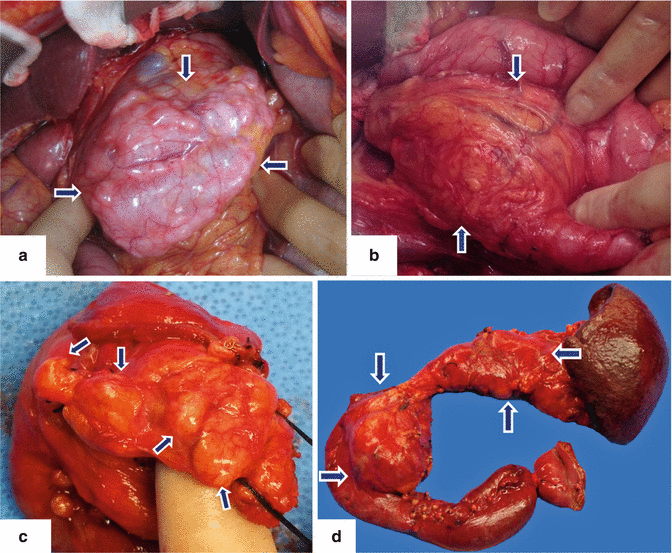
Fig. 8.3
IPMN gross appearance. Intraoperative photographs with proven diagnosis of IPMNs demonstrate: a: a tortuous mass of multiple, small cysts in the body of the pancreas (arrows). b: a large, unilocular mass in the head of the pancreas adjacent to the duodenum (arrows). Photographs of a surgical specimen from a Whipple procedure (c) demonstrates a multilobulated mass in the head of the pancreas (arrows) and a lobulated cystic mass (d) (arrows) in the head and body of the pancreas from a total pancreatectomy
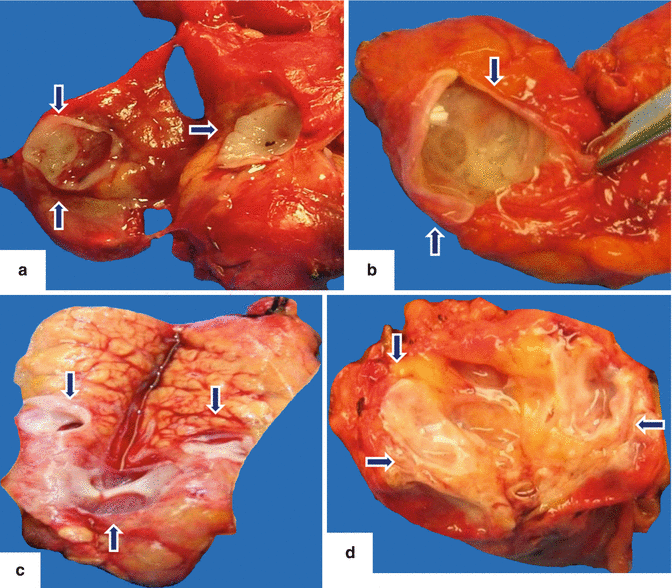
Fig. 8.4
IPMN internal appearance. Photographs of bivalved side-branch type IPMNs reveal: a: small, unilocular cystic mass (arrows). b: mid-size, unilocular cystic mass with mucoid material and undulating inner surface (arrows). c: mass composed of a few small cysts separated by fibrous septa (arrows). d: multicystic mass filled with mucoid material (arrows)
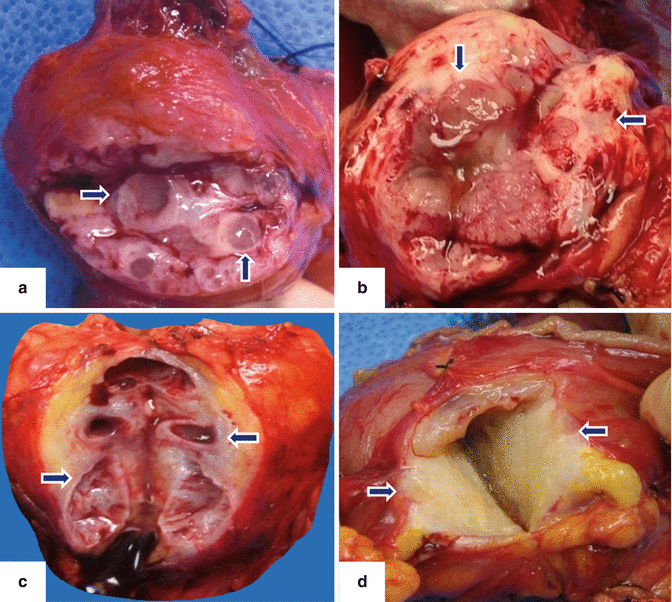
Fig. 8.5
IPMN internal appearance. Photographs of bivalved side-branch type IPMNs show: a: cystic mass composed of small cysts filled with mucin (arrows). b: ill-defined, partially cystic mass with hemorrhage, mucin, and fibrosis (arrows). c: oligocystic mass with smooth inner lining and fibrosis (arrows). d: ill-defined, yellow, solid mass with areas of fibrosis (arrows)
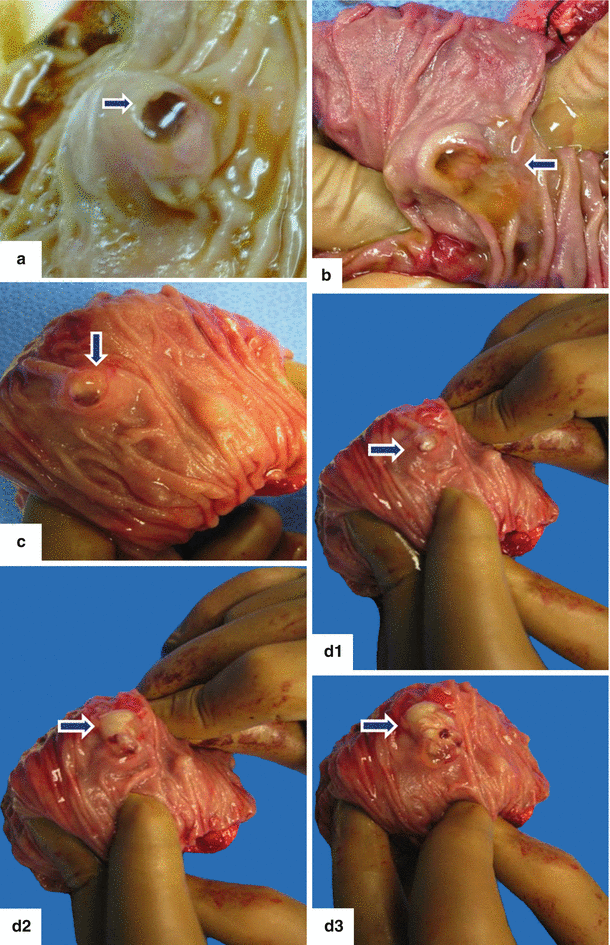
Fig. 8.6
Major duodenal ampulla appearance in IPMN. Photographs of the ampulla of Vater with IPMNs. a–c: reveal a patulous ampulla with fish eye appearance (arrows) and viscous mucin protruding into the duodenum (arrows). d: photographs (d1–d3) show inspissated mucus being extruded from an IPMN through the ampulla of Vater into the duodenum (arrows)
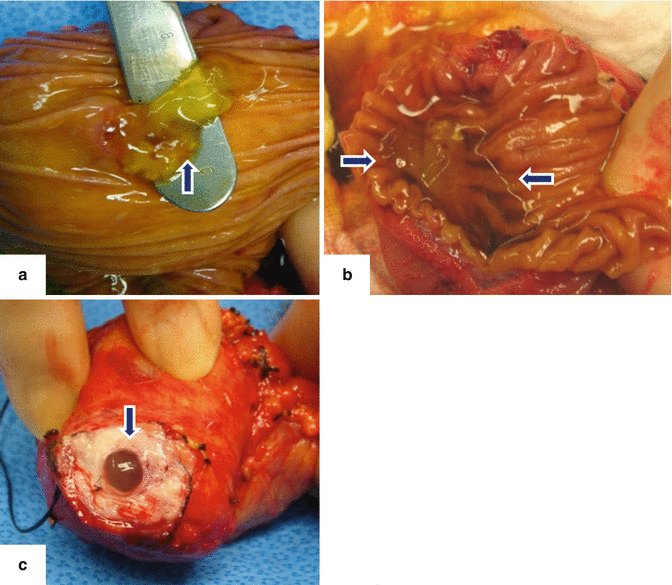
Fig. 8.7
Photographs show the different mucoid material produced by the IPMNs in different locations. a: protruding from the ampulla of Vater (arrow). b: within the duodenum (arrows). c: protruding from the main pancreatic duct (arrow)
Branch duct-type (BD) IPMN
Single or multiloculated cyst with intraluminal mucin
Communicates with the pancreatic duct system
Shows atrophy of adjacent pancreatic parenchyma
Main pancreatic duct-type (MD) IPMN
Florid papillary projections in dilated main pancreatic duct
May involve only a portion of the duct resulting in a unilocular cystic mass due to mucin expansion
May result in diffuse distention of the pancreatic duct with parenchymal atrophy
Combined-type (C) IPMN
Combination of both
Practical Pearls
Pathologists should make every attempt to classify a lesion as MD-IPMN or BD-IPMN; being careful to identify the main pancreatic duct as accurately as possible when processing a specimen.
8.4.2 Microscopic Characteristics
IPMNs are pancreatic cystic masses with malignant potential.
Show dysplasia – adenocarcinoma sequence.
IPMNs are mucinous neoplasms that lack a distinctive ovarian-type stroma.
Histologically, it may demonstrate areas of varying degrees of dysplasia, low- to intermediate- or high-grade, carcinoma in situ, or invasive, within the same tumor.
Low-grade dysplasia: mild cytologic atypia and no architectural atypia. Tall, columnar, uniform cells with round to oval, basally located nuclei and abundant apical mucin.
Intermediate-grade dysplasia: mild to moderate cytologic atypia. Columnar cells show nuclear polarization and stratification, variable mucin content, and high nuclear to cytoplasmic ratio.
High-grade dysplasia/carcinoma in situ: architectural complexity with crowded, branching, and budding papillae. Marked nuclear atypia with mucin depletion and mitotic activity.
IPMNs are graded based on the most severe histologic finding.
Up to 35 % of IPMNs have associated invasive carcinoma.
Invasive adenocarcinomas show tubular features reminiscent of conventional ductal adenocarcinomas or colloid features.
8.4.3 IPMNs Epithelial Subtypes (Fig. 8.8)
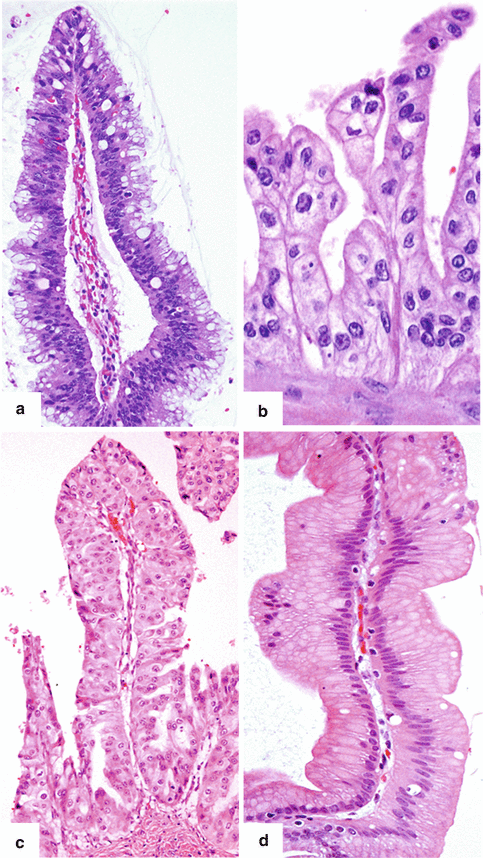
Fig. 8.8
IPMN epithelium subtypes. (a) Intestinal: papilla lined by epithelial cells with basophilic cytoplasm, enlarged oval hyperchromatic nuclei, pseudostratification, and abundant goblet cells. (b) Pancreatobiliary: thin branching papillae lined by cuboidal to columnar cells wtih amphophilic cytoplasm and enlarged hyperchromatic nuclei. (c) Oncocytic: thick branching papilla lined by cells with large, round nuclei, prominent nucleoli, and the characteristic abundant oncocytic cytoplasm. (d) Gastric/Foveolar: papilla lined by columnar epithelium with basally located nuclei and abundant amount of slightly eosinophilic mucinous cytoplasm (H&E, 40×, 60×)
Four subtypes of epithelia can be identified in IPMNs
Intestinal
Pancreaticobiliary
Oncocytic
Gastric
Intestinal epithelial subtype (Fig. 8.8a)
Most common subtype of MD-IPMN
Typically occurs in the pancreatic head and may involve the entire pancreatic duct
Growth pattern: villous
Expresses mucin 2 (MUC2), MUC5, and caudal-type homeobox 2 (CDX2)
When invasive: mucinous (colloid) adenocarcinoma
Pancreatobiliary epithelial subtype (Fig. 8.8b)
Typically involves the main duct in the pancreatic head.
Produces less mucus than intestinal-type IPMN.
Complex arborizing papillae.
Cells resemble pancreatic and biliary duct cells.
Expresses MUC1, MUC5 (focal), and weak MUC6.
When invasive: ductal (tubular) adeno-carcinoma.
Oncocytic epithelial subtype (Fig. 8.8c)
Rare.
Forms large tissue nodules in the main pancreatic duct.
Produces little mucus.
Composed of oncocytic papillae with complex branching pattern.
Cytoplasm of the lining cells is eosinophilic.
Expresses MUC 5 AC and MUC6 and may show variable and focal expression of MUC1.
When invasive: ductal (tubular) adenocarcinoma.
Gastric epithelial subtype (Fig. 8.8d)
Most common IPMN subtype
Found in side-branch IPMN
Occurs in the periphery of pancreatic parenchyma
Often found in uncinate process
Papillae are lined by epithelium resembling gastric foveolar cells
May form pyloric gland structures at the base of the papillae
Express MUC5AC and MUC6
Do not express or are only focally positive for MUC1 and MUC2
When invasive (rarely): ductal (tubular) adenocarcinoma
8.4.4 Molecular Abnormalities Associated with IPMNs
K-ras mutations in approximately 50 % of the cases
Inactivating mutations in the ring finger protein 43 (RNF43)
Mutations in STK11 (Peutz-Jeghers)
MUC2 mucin and MUC5 mucin mRNA
Mutations in the tumor suppressor gene CDKN2a
Hypermethylation of promoter sequences
Telomerase reverse transcriptase (hTERT) and Sonic Hedgehog (SHh)
8.5 Clinical Presentation
Most of these cystic tumors are detected incidentally by imaging (CT/MR).
Some patients may present with:
Abdominal pain
Back pain
Jaundice
Weight loss
Anorexia
Nausea
Vomiting
Repetitive attacks of pancreatitis (epigastric pain)
Acute onset of diabetes mellitus
Exocrine pancreatic insufficiency (diarrhea, steatorrhea)
Practical Pearls
The history of repetitive attacks of acute pancreatitis in elderly males should alert the clinician to the possible etiology of obstruction of the major papilla secondary to mucin material produced by an IPMN. Further evaluation with MRCP is recommended.
The presence of clinical symptoms associated with an IPMN should raise the possibility of malignant transformation.
8.6 Laboratory Evaluation
In most cases, routine laboratory tests are normal.
Episodes of pain may promote elevation of pancreatic enzymes (amylase and lipase).
Serum levels of carbohydrate antigen (CA-19.9) and carcinoembryonic antigen (CEA) are used to differentiate invasive from noninvasive IPMN.
Pancreatic cyst fluid analysis:
CEA is the best marker.
CEA >192 ng/ml (high sensitivity and specificity).
Helpful to distinguish mucinous from non-mucinous cysts.
8.7 Imaging
Magnetic resonance (MR) or contrast-enhanced computed tomography (CECT) is the initial test recommended to evaluate patients with suspected IPMN.
MR of the pancreas in combination with MRCP and endoscopic ultrasound (EUS) is considered the study of choice for following up IPMNs.
Transabdominal ultrasound is usually limited for diagnosing IPMN.
When the imaging diagnosis of an IPMN is questionable, EUS with fine-needle aspiration (FNA) for cytology analysis and determination of CEA and CA 19.9 levels is a very useful tool for establishing a definitive diagnosis.
Some centers use intraductal ultrasound, ERCP, and pancreatoscopy in order to distinguish between benign and malignant tumors.
The sensitivity of these methods is lower for side-branch lesions compared to lesions along the main pancreatic duct.
PECT/CT is indicated for the detection of malignant IPMNs in select cases.
8.7.1 Ultrasound (Figs. 8.9–8.13)
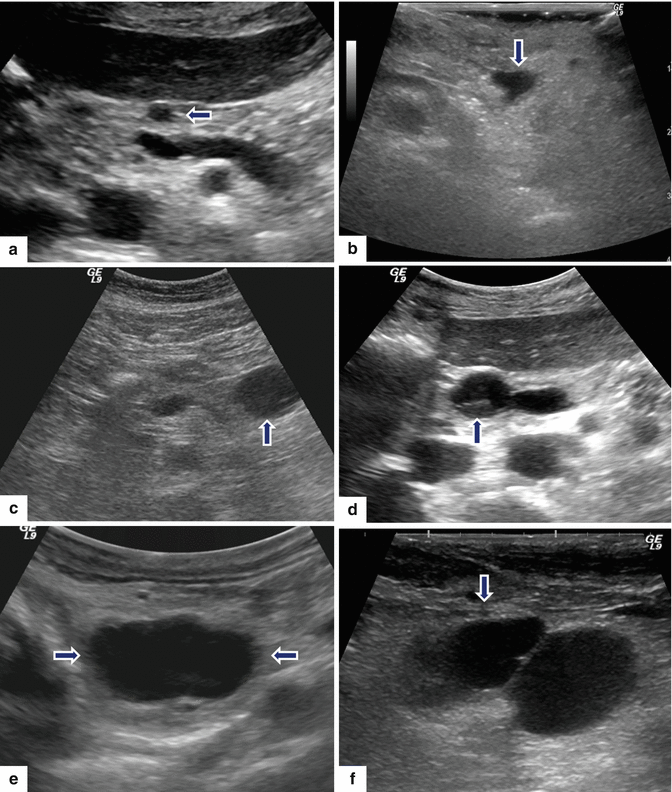
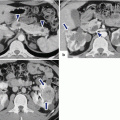
Fig. 8.9
Nonmalignant side-branch-type IPMNs, spectrum of appearances on ultrasound. Conventional ultrasound transverse images in four patients (a–d) show: (a) small, round, unilocular cystic mass (arrow), (b) small, unilocular cystic mass with lobulated margins (arrow), (c) small cystic mass with internal septation (arrow), and (d) small cystic mass with small papillary projection (arrow). Intraoperative ultrasound transverse images in two patients show: (e) large, ovoid, unilocular mass (arrows) and (f) bilobulated cystic mass (arrow)
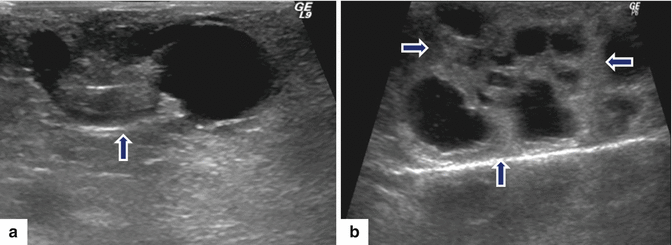
Fig. 8.10
Malignant side-branch type IPMNs on ultrasound. Intraoperative ultrasound transverse images in two patients. a: shows a complex ovoid mass with cystic and solid components (arrow). b: shows a multilocular cystic mass with thick septa (arrows)
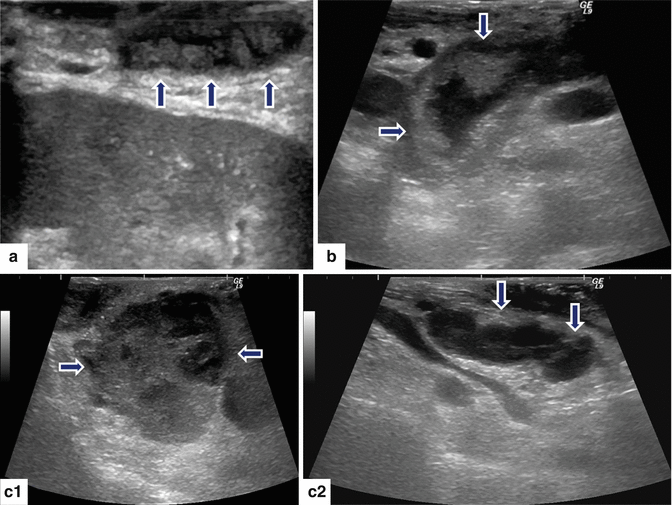
Fig. 8.11
Malignant main duct IPMNs appearance on intraoperative ultrasound (IOUS). Transverse images in three patients demonstrate: a: dilated main pancreatic duct with multiple internal mural nodules (arrows). b: markedly dilated main pancreatic duct with internal projections (arrows). c: c1–c2 images: a large, solid mass with peripheral small cystic components in the head of the pancreas (c1) (arrows) associated with a dilated main pancreatic duct containing internal projections (c2) (arrows)
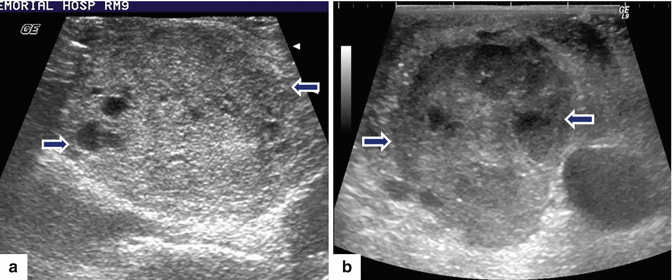
Fig. 8.12
IPMNs solid appearance on intraoperative ultrasound (IOUS). Intraoperative ultrasound transverse images (a, b) demonstrate a round, well-demarcated, mostly solid mass with small cystic components in the head of the pancreas (arrows)
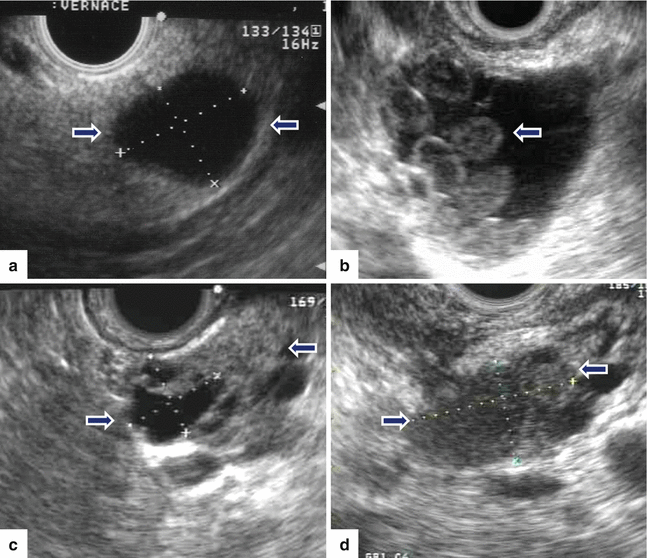
Fig. 8.13
IPMNs appearance on endoscopic ultrasound (EUS) in four patients. Transverse images demonstrate: a: a unilocular cystic mass (arrows). b: a cystic mass with internal projections (arrow). c: a complex mass with solid and cystic components (arrows). d: a mostly solid, heterogeneous mass (arrows)
Findings
Transabdominal ultrasound (TAUS)
Unilocular round or ovoid cystic mass
Multiloculated cystic mass
Complex mass (cystic and solid components)
Diffuse or focal dilatation of the main pancreatic duct
Endoscopic ultrasound (EUS) and intraoperative ultrasound (IOUS)
Branch duct-type IPMN
Unilocular cystic mass or multicystic mass composed of small cysts (5–20 mm) that communicate with the pancreatic duct system
Mural nodules
Complex mass (solid and cystic components)
Main duct-type IPMN
Segmental or diffuse dilatation of the main pancreatic duct
Intraductal mural nodules
Intraductal ultrasound (IDUS)
Useful to determine tumor extent in MD-type IPMN
Practical Pearl
The availability of intraductal ultrasound is limited in most medical centers. It requires sophisticated equipment and an expert operator.
8.7.2 Contrast-Enhanced Computed Tomography (CECT) and Magnetic Resonance Imaging (MRI) (Figs. 8.14–8.68)
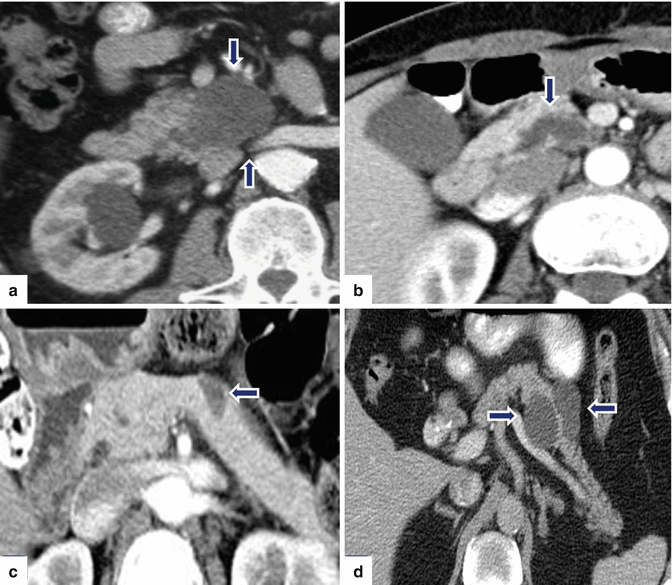
Fig. 8.14
Nonmalignant side-branch type IPMNs, spectrum of appearances on CT. CECT axial images demonstrate: Patient a: a multilocular cystic mass in the uncinate process (arrows). Patient b: a tubular-like appearance cystic mass in the uncinate process (arrow). Patient c: a unilocular cystic mass in the body of the pancreas (arrow). Patient d: a bilobulated cystic mass in the head of the pancreas (arrows)
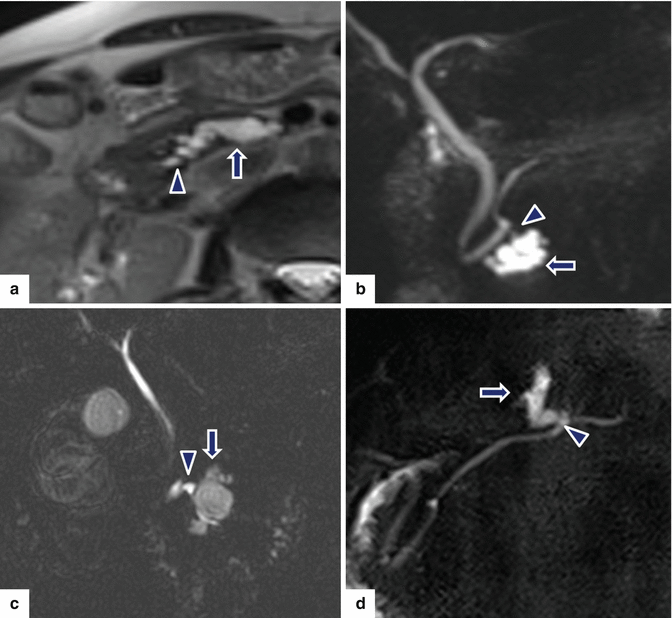
Fig. 8.15
Nonmalignant side-branch type IPMNs, spectrum of appearances on MR. a: single-shot fast spin echo T2-weighted axial image shows a cystic mass with finger-like tubular appearance involving the uncinate process (arrow). b: MRCP thick slab image shows a mass composed of multiple small cysts (arrow). c: MRCP thick slab image shows a multilocular cystic mass in the uncinate process (arrow). d: MRCP thick slab image shows a tubular cystic mass involving the body of the pancreas (arrow). a–d: note the communication of each mass with the main pancreatic duct (arrowheads)
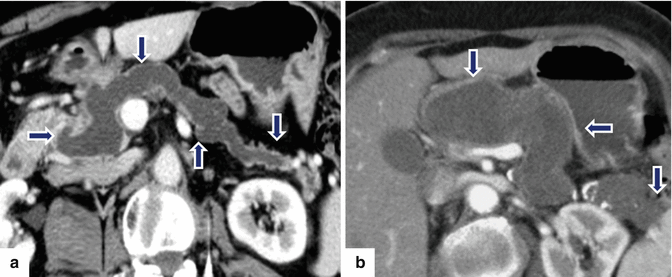
Fig. 8.16
Main duct-type IPMNs appearance on CT. CECT axial images show: Patient a: diffuse, dilated pancreatic duct (arrows). Patient b: markedly diffuse, dilated main pancreatic duct with intraluminal low-density amorphous material (arrows). Patients a, b: note the atrophy of the pancreatic parenchyma associated with each mass
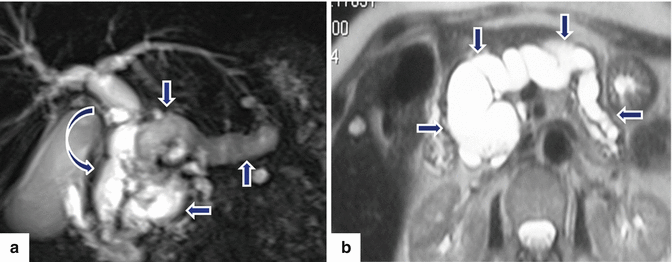
Fig. 8.17
Main duct-type IPMNs appearance on MR. a: MRCP coronal image (MIP) reveals a markedly diffuse dilated main pancreatic duct (arrows) and associated diffuse dilatation of the intra- and extrahepatic biliary system (curved arrow). b: single-shot fast spin echo T2-weighted axial image reveals a diffuse, markedly dilated main pancreatic duct (arrows). a, b: note the diffuse atrophy of the pancreatic parenchyma associated with each mass
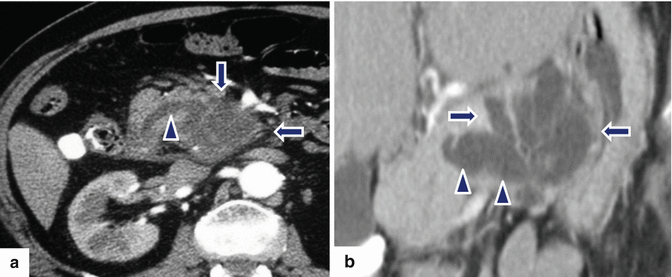
Fig. 8.18
Combined-type IPMNs appearance on CT. CECT axial images show: Patient a: a multilocular cystic mass in the uncinate process (arrows) extending into the main pancreatic duct (arrowhead). Patient b: a cluster of dilated tubular structures in the body of the pancreas (arrows) extending into the main pancreatic duct (arrowheads)
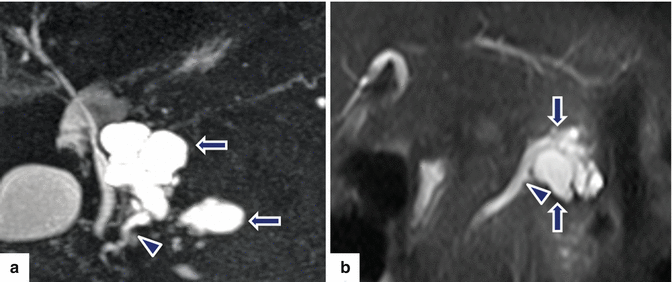
Fig. 8.19
Combined-type IPMNs appearance on MR. Patient a: MRCP thick slab image shows two lobulated cystic masses in the uncinate process (arrows) extending into the main pancreatic duct (arrowhead). Patient b: single-shot fast spin echo axial image shows a multilocular cystic mass in the body of the pancreas (arrows), extending into the main pancreatic duct (arrowhead)
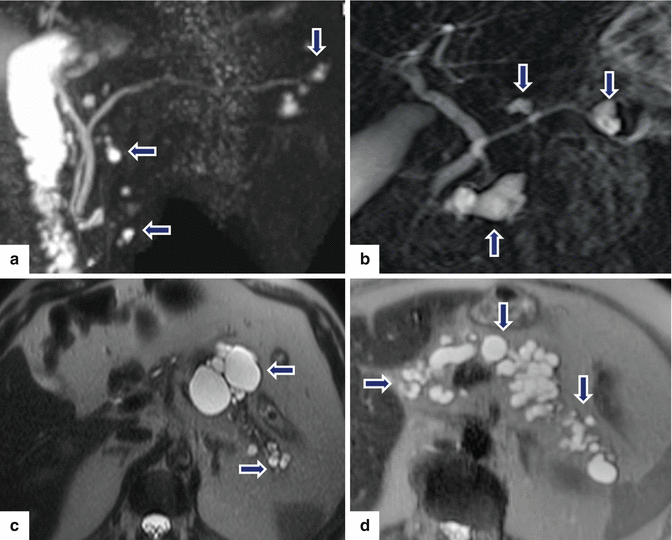
Fig. 8.20
Multifocal IPMNs appearance on MR. Patients a, b: MRCP thick slab images show multiple, small cystic masses throughout the pancreas (arrows). Patients c, d: single-shot fast spin echo T2-weighted images show multiple, small cystic masses throughout the pancreas (arrows)
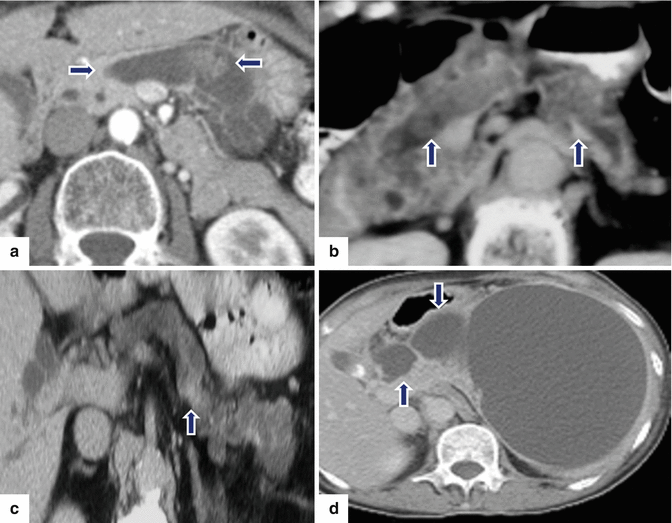
Fig. 8.21
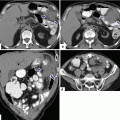
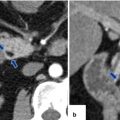
Findings suggestive of IPMNs malignant transformation on CT. CECT axial images of four patients: Patient a: dilated main pancreatic duct with a mural nodule (arrows). Patient b: dilated main pancreatic duct with multiple intramural nodules (arrows). Patient c: focal area of pancreatic parenchyma enhancement (arrow) associated with a dilated main pancreatic duct and atrophy of the pancreas. Patient d: large cystic pancreatic mass extending into the main pancreatic duct (arrows)
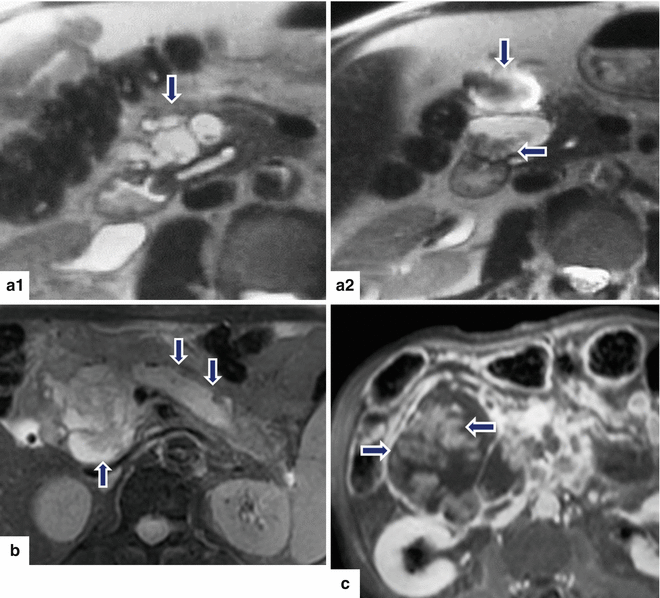
Fig. 8.22
Findings suggestive of IPMNs malignant transformation on MR. MR images of three patients. Patient (a): single-shot fast spin echo T2-weighted axial images (a1–a2) show a large multilocular cystic mass with small internal mural nodules (arrows). Patient b: fat-suppressed T2-weighted axial image shows a complex cystic mass with thick, irregular low signal papillary growths (arrows) associated with dilatation of the pancreatic duct. Patient c: fat-suppressed contrast-enhanced T1-weighted axial image shows a large cystic mass with enhancing papillary nodules involving the head of the pancreas (arrows)
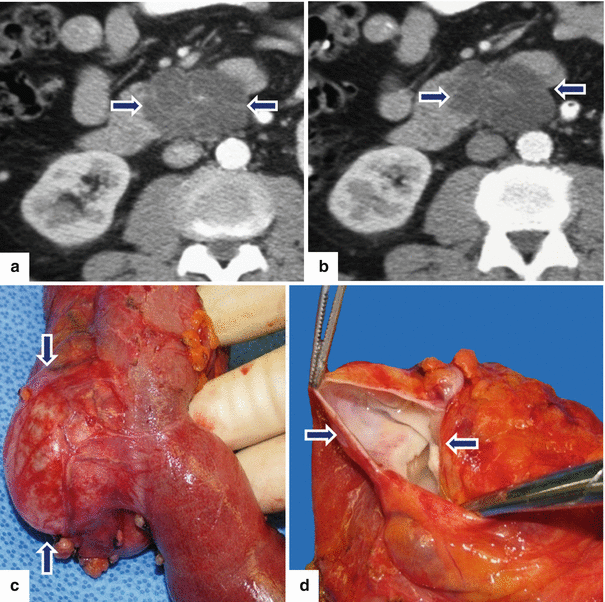
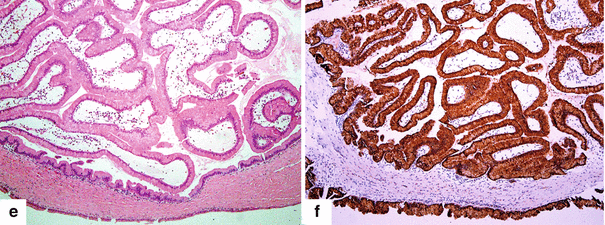
Fig. 8.23
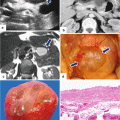
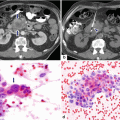
Side-branch type, borderline IPMN on CT. A 79-year-old male with a 2-year history of a cystic lesion of the pancreas that had been followed by serial CTs. On the last study, it was noted that the lesion had doubled in size. Patient had also experienced a 10 lb weight loss. CECT axial images (a, b) show a multilobulated cystic mass involving the head and uncinate process of the pancreas (arrows). Photograph of the resected gross specimen (c) shows a well-defined, ovoid mass with smooth serosal surface (arrows) adjacent to the duodenum. Photograph of the bisected ovoid mass (d) shows a cystic tumor with smooth internal lining and thin fibrous septa (arrows). On microscopic examination (e) (H&E, 10×) the cyst was lined by benign foveolar-type epithelium with tall columnar cells, basally located nuclei, and abundant mucinous cytoplasm. The tumor cells are strongly positive for MUC-5 (f) by immunohistochemistry (immunostain, 10×)
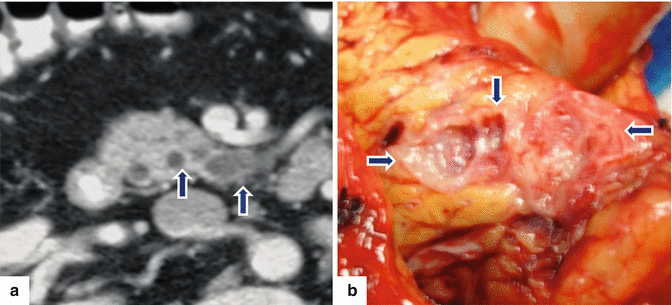
Fig. 8.24
Side-branch type adenoma IPMN on CT and MR. A 67-year-old male complaining of mild epigastric pain. A conventional abdominal ultrasound (not shown) revealed a mass in the head of the pancreas. The patient underwent an endoscopic ultrasound with fine-needle aspiration that suggested a mucinous pancreatic tumor. CECT axial image (a) shows a small multicystic mass (arrows) involving the uncinate process. The patient underwent a pyloric-sparing Whipple procedure. Photograph of the gross specimen (b) shows a multilocular cystic mass in the head of the pancreas (arrows)
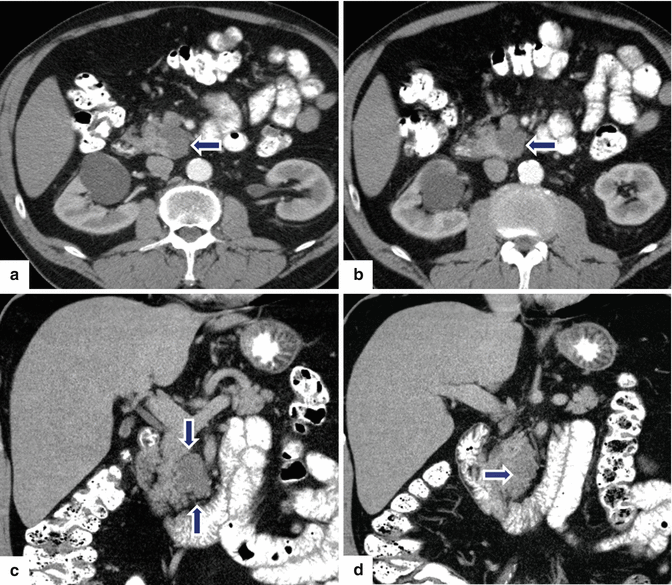
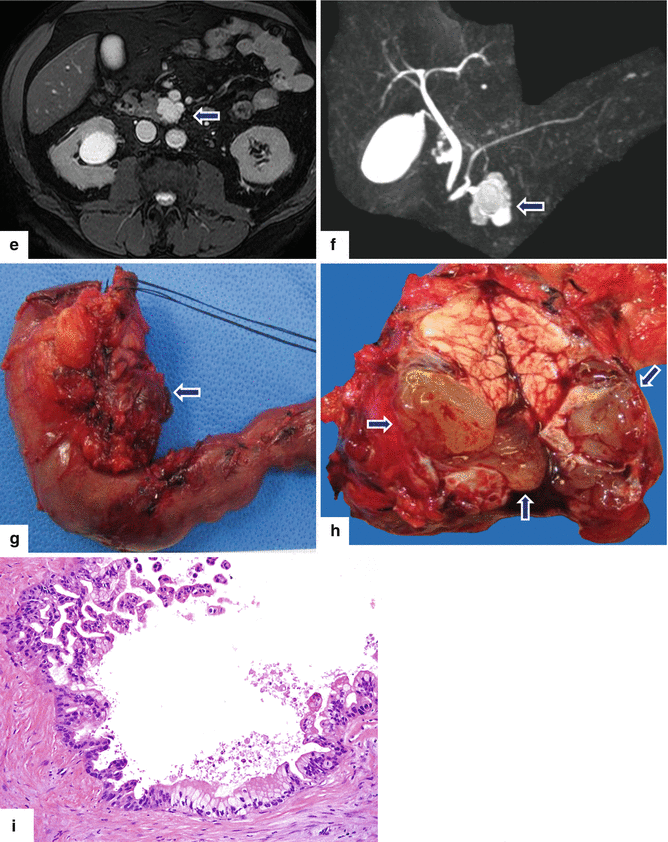
Fig. 8.25




Side-branch type IPMN on CT. A 63-year-old male with an incidental finding on a CT of the abdomen performed to evaluate a complicated inguinal hernia revealing a pancreatic mass. Endoscopic ultrasound with fine-needle aspiration showed a 35 mm cystic lesion with internal septa containing a solid component and other areas with possible papillary formations and a viscous, mucinous fluid, difficult to aspirate. No communication with the pancreatic duct was demonstrated. CECT axial (a, b) and coronal (c, d) images show a multilobular cystic mass in the uncinate process of the pancreas (arrows). T2-weighted axial image (e) shows a mass with high signal intensity (arrow), and MRCP reformatted coronal image (f) shows a multicystic mass in the uncinate process communicating with pancreatic duct (arrow). The patient underwent a pyloric-sparing Whipple procedure. Photograph of the surgical specimen (g) shows a lobulated mass (arrow). Photograph of the sectioned gross specimen (h) shows a cystic mass filled with a thick mucinous material (arrows). Final pathology (i): IPMN with intermediate- to high-grade dysplasia, side-branch type (H&E, 20×)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree