Abstract
There are multiple etiologies for fetal growth restriction, and a thorough patient history and physical examination are important to identify risk factors for the current pregnancy and future reproductive risks. Ultrasound (US) estimated fetal weight less than the 10th percentile for gestational age is the most widely used parameter, though greater sensitivity for the diagnosis is seen with stricter parameters. A targeted US is necessary to diagnose fetal anomalies. Genetic counseling and amniocentesis for the diagnosis of aneuploidy or infection can be offered to the patient, especially if there are associated fetal structural abnormalities. Serial US for growth and amniotic fluid, Doppler velocimetry, and prenatal testing are useful in determining the appropriate timing of delivery. There are both short-term and long-term consequences of fetal growth restriction.
Keywords
fetal growth restriction, Doppler, intrauterine growth restriction
Introduction
Intrauterine growth restriction (IUGR) is a significant pregnancy complication that has both short-term and long-term implications for the fetus and the neonate. A growth-restricted fetus is at increased risk of perinatal morbidity and mortality, and the risks are carried into adulthood with an increased risk of cardiovascular disease, hypertension, obesity, diabetes, and osteoporosis, regardless of the gestational age at delivery.
Disease
Definition
IUGR is generally defined as an estimated fetal weight at or below the 10th percentile. There is a direct relationship between fetal growth and adverse outcomes with the greatest risk of perinatal mortality and morbidity at weights below the third percentile. Correctly diagnosing IUGR is essential in managing pregnancy and hinges on the establishment of accurate dating criteria as early as possible.
Prevalence and Epidemiology
The prevalence of IUGR is 10% by definition, although the population studied, geographic location, altitude, and growth curves that are used all affect rates. Controversy exists because many fetuses classified as growth restricted are constitutionally small and healthy and are not at risk for adverse outcomes, whereas others who may be above the 10th percentile but are not achieving their growth potential, are at higher risk for complications. It is important to use growth curves specific for the population being followed. Higher rates are seen in developing countries because of the underlying disease burden and malnutrition.
Genetic factors, including gender, significantly contribute to the variation seen in birth weights. Maternal genes influence birth weight more than the paternal genome. Maternal birth weight and a history of a prior growth-restricted infant or poor pregnancy outcome increase the risk of subsequent pregnancies having growth restriction. Higher rates are seen with advancing maternal age and with chronic medical diseases.
Approximately 25% of multiple gestations are growth restricted, with the highest rates seen in monochorionic gestations. Growth restriction is observed in 20% to 25% of fetuses with congenital anomalies and in 20% of stillborns. Fetal mortality is increased because of the underlying etiology and associated preterm deliveries. Perinatal mortality rates are four to eight times higher for growth-restricted infants, and morbidity is present in 50% of surviving infants. Male fetuses with IUGR have a higher overall mortality than female fetuses.
Etiology and Pathophysiology
There are multiple etiologies associated with IUGR, which can be intrinsic or extrinsic to the fetus ( Table 110.1 ). Any disease that affects the vascular endothelium and the uteroplacental circulation has the potential to affect nutrient delivery.
| MATERNAL |
|
| FETAL |
|
| PLACENTA |
|
The uterine blood supply derives mainly from the uterine arteries, which give rise to the arcuate arteries that run circumferentially around the uterus. The radial arteries arise from arcuate vessels and penetrate at right angles into the outer third of the myometrium, giving rise to the basal and spiral arteries, which supply the myometrium, decidua, and intervillous space. The first-trimester placenta is an organ with high impedance to blood flow. Physiologic modification of the spiral arteries is necessary to allow the 10-fold increase in uterine circulation necessary for fetal growth. This increase occurs in the second wave of trophoblastic invasion when the spiral arteries are invaded by cytotrophoblast cells and are converted into uteroplacental arteries. Normal uteroplacental arteries have dilated and tortuous lumina, a noncontinuous endothelial lining, and complete absence of muscular and elastic tissue, which converts the placenta into a low-resistance organ. Fewer spiral arteries, less branching, and occlusion of the lumina characterize the growth-restricted placenta, with additional wall thickening and fewer gas-exchanging terminal villi, which are poorly branched. The failure of normal development of maternal placental arteries leads to reduced oxygen and nutrient delivery into the intervillous space, whereas reduction in the number of placental terminal capillaries leads to poor transfer of oxygen and nutrients to the fetus.
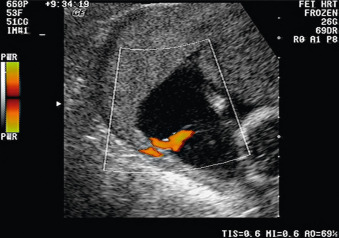
If the physiologic conversion to low resistance develops appropriately, there is high-velocity diastolic blood flow in the uterine arteries in the latter part of the second trimester and the early diastolic notching of the uterine artery Doppler signal, which is present in the nonpregnant state and early pregnancy, disappears ( Figs. 110.1 and 110.2 ). If the physiologic conversion has not occurred, uterine artery blood flow and Doppler patterns present in the first trimester persist into the second and third trimesters, with high systolic-to-diastolic (S/D) ratios, notching, and reversal of flow ( Fig. 110.3 ). The occurrence of the physiologic change has been used as a screening tool for IUGR and pregnancy complications in high- and low-risk pregnancies. Abnormalities in flow in the uterine arteries are associated with higher incidence of IUGR, oligohydramnios, preeclampsia, emergency cesarean delivery, abruption, shorter duration of pregnancy, and poorer neonatal outcome. Elevated uterine artery Doppler indices at 10 to 14 weeks’ gestation also have been shown to be predictive of IUGR and pregnancy complications, whether used alone or in combination with maternal history.



Fetal growth aberrations have been classified into symmetric and asymmetric (or head sparing). Normally, abdominal circumference increases in the third trimester because of increasing adiposity and an increase in liver glycogen stores and size. Symmetric growth restriction, which accounts for 20% to 30% of growth-restricted fetuses, refers to a growth pattern in which all fetal organs and growth parameters are decreased proportionally, secondary to impairment of early fetal cell growth. Etiologies include genetic or chromosomal causes, early infections, drug or alcohol exposure, and severe early placental insufficiency . Asymmetric growth restriction is characterized by a decrease in abdominal circumference relative to the head circumference. This pattern results from the fetus using nutritional stores in the liver and adapting blood flow to compensate for inadequate placental perfusion. The redistribution of blood flow to more vital organs (brain, heart, and adrenals) at the expense of less vital organs (intestines, muscle, kidneys, and lungs) leads to oligohydramnios from decreased renal perfusion and urine output. The decreased tissue perfusion contributes to fetal acidemia and causes direct damage to the deprived tissues. Blood flow alterations as assessed by Doppler velocimetry and oligohydramnios precede changes in fetal heart rate reactivity and variability.
These ultrasound (US) findings strengthen the diagnosis of IUGR and indicate an increased likelihood of perinatal mortality and morbidity. Progressive compensation by the fetus leads to decreased diastolic flow and eventually absent or reversed end-diastolic flow in the umbilical artery. Both signs are highly predictive of perinatal mortality. Progressive hypoxemia and acidemia lead to cardiac dysfunction and myocardial ischemia. Tricuspid regurgitation and right-sided heart failure can be detected as venous backflow by sampling the inferior vena cava, ductus venosus, and umbilical vein. Venous Doppler changes occur in more than half of cases before an abnormal biophysical profile or nonstress test. Umbilical vein pulsations should be considered a near-terminal event.
Manifestations of Disease
Clinical Presentation
The most important tool in the diagnosis of fetal growth restriction is a thorough assessment of underlying risk factors. In the absence of baseline risk factors, fundal height measurements, weight gain or loss, and the development of pregnancy-related complications are followed during the course of routine prenatal care to determine the need for further assessment by US. Routine screening of uterine and umbilical artery Doppler has not been shown to reduce maternal or perinatal morbidity and mortality in a low-risk population. Patients with underlying risk factors should be followed by serial assessments of growth and amniotic fluid volume.
Imaging Technique and Findings
Ultrasound.
US is the main diagnostic modality for both screening and determination of IUGR. The presence of a fetal or placental abnormality at any gestational age warrants follow-up assessment of growth and amniotic fluid ( Figs. 110.4 and 110.5 ). The discovery of growth restriction by US at any gestational age requires a detailed anatomic survey for underlying fetal etiologies and a detailed maternal history. Genetic counseling and amniocentesis for the diagnosis of aneuploidy or infection can be offered to the patient, especially if there are associated fetal structural abnormalities.