Chapter 2 Intravascular contrast media
Historical Development of Radiographic Agents
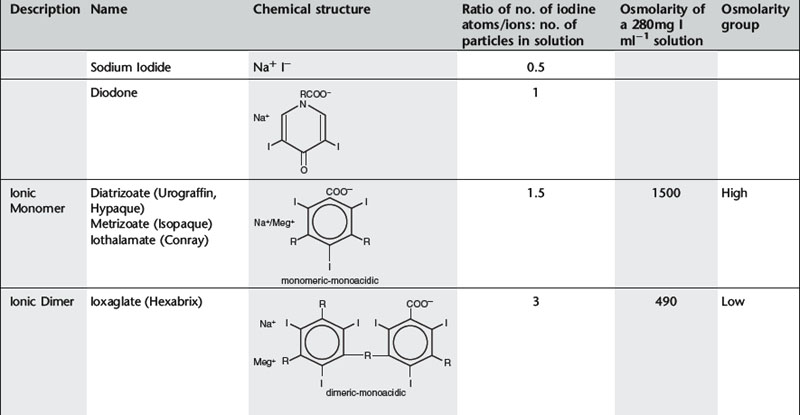
The first report of opacification of the urinary tract after intravenous (i.v.) injection of a contrast agent appeared in 1923, when Osborne et al. took advantage of the fact that i.v.-injected, 10% sodium iodide solution, which was then used in the treatment of syphilis, was excreted in the urine. In 1928 German researchers synthesized a compound with a number of pyridine rings containing iodine in an effort to detoxify the iodine. This mono-iodinated compound was developed further into di-iodinated compounds and subsequently in 1952 the first tri-iodinated compound, sodium acetrizoate (Urokon), was introduced into clinical radiology. Sodium acetrizoate was based on a 6-carbon ring structure, tri-iodo benzoic acid, and was the precursor of all modern water-soluble contrast media.
Until the early 1970s all contrast media were ionic compounds and were hypertonic with osmolalities of 1200–2000 mosmol kg−1 water, 4–7 × the osmolarity of blood. These are referred to as high osmolar contrast media (HOCM) and are distinguished by differences at position 5 of the anion and by the cations sodium and/or meglumine. In 1969 Almén first postulated that many of the adverse effects of contrast media were the result of high osmolality and that by eliminating the cation, which does not contribute to diagnostic information but is responsible for up to 50% of the osmotic effect, it would be possible to reduce the toxicity of contrast media.1
Conventional ionic contrast media had a ratio of three iodine atoms per molecule to two particles in solution, i.e. a ratio of 3:2 or 1.5 (Table 2.1). In order to decrease the osmolality without changing the iodine concentration, the ratio between the number of iodine atoms and the number of dissolved particles must be increased.
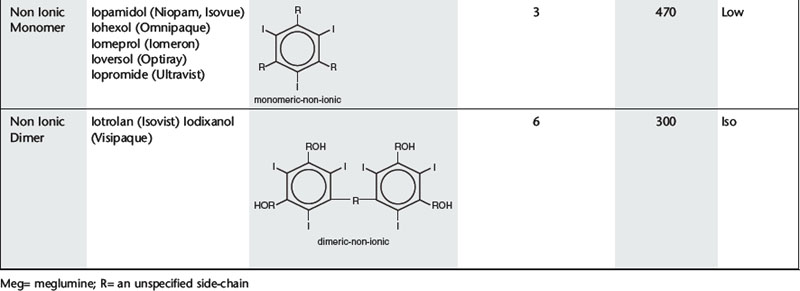
Further development proceeded along two separate paths (Table 2.1). The first was to combine two tri-iodinated benzene rings to produce an ionic dimer with six iodine atoms per anion, the low osmolar contrast medium (LOCM) ioxaglate (Hexabrix). Replacement of one of the carboxylic acid groups with a non-ionizing radical means that only one cation is needed per molecule. The alternative, more successful, approach was to produce a compound that does not ionize in solution and so does not provide radiologically useless cations. Contrast media of this type are referred to as non-ionic and are also LOCM. These include the non-ionic monomers iopamidol (Niopam, Iopamiron, Isovue, and Solutrast), iohexol (Omnipaque), iopromide (Ultravist), iomeprol (Iomeron) and ioversol (Optiray).
For both types of LOCM the ratio of iodine atoms in the molecule to the number of particles in solution is 3:1 and osmolality is decreased. Compared with high osmolar contrast material (HOCM), the LOCM show a theoretical halving of osmolality for equi-iodine solutions. However, because of aggregation of molecules in solution the measured reduction is approximately one-third (Fig. 2.1).
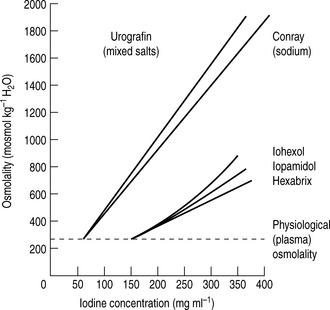
Figure 2.1 A plot of osmolality against iodine concentration for new and conventional contrast media. From Dawson et al.2
(Reproduced by courtesy of the editor of Clinical Radiology.)
A further development in the search for the ideal contrast agent has been the introduction of non-ionic dimers – iotrolan (Isovist) and iodixanol (Visipaque). These have a ratio of six iodine atoms for each molecule in solution with satisfactory iodine concentrations at iso-osmolality; they are, therefore, called iso-osmolar contrast media. The safety profile of the iso-osmolar contrast agents is at least equivalent to LOCM, but any significant advantage of iso-osmolar contrast remains controversial.
The low- and iso-osmolar contrast media are 5–10 times safer than the HOCM.3 Previously, HOCM were much less expensive than LOCM so were widely used despite the higher risk of adverse reaction and nephrotoxicity. LOCM were reserved for use in patients considered to be at increased risk of contrast reaction. Over the past few years, the relative cost of the non-ionic contrast media has fallen and the use of ionic contrast medium for i.v. injection has now ceased almost entirely. Ionic contrast media must never be used in the subarachnoid space.
ADVERSE EFFECTS OF INTRAVENOUS WATER-SOLUBLE CONTRAST MEDIA
Adverse reactions after administration of non-ionic iodinated contrast media are rare, occuring in less than 1% of all patients.4 Of these reactions, the majority are mild and self-limiting. The incidence of severe or very severe non-ionic contrast reaction is 0.044%.5
TOXIC EFFECTS ON SPECIFIC ORGANS
Vascular toxicity
Venous
Cardiovascular toxicity
Haematological changes
Nephrotoxicity
The mechanisms of CIN are summarized below:10
It was hoped that the iso-osmolar contrast agents might be less nephrotoxic than LOCM and HOCM. However, clinical trials have so far yielded conflicting results.9,11 CIN has a complex aetiology and the positive benefit of reduction in osmolarity achieved with iso-osmolar contrast medium may be negated by the accompanying increase in viscosity.12
See Table 2.3 for guidelines on prophylaxis of renal adverse reaction to iodinated contrast.
Neurotoxicity
Convulsions may also occur secondary to the cerebral hypoxia caused by hypotension ± cardiac arrest after severe contrast reaction.
IDIOSYNCRATIC REACTIONS
Excluding death, adverse reactions can be classified in terms of severity as:
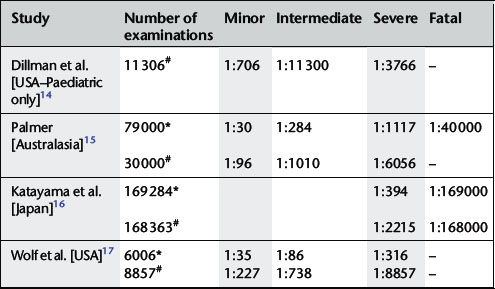
Minor and intermediate reactions are not uncommon; major adverse reactions are rare (Table 2.2).
Fatal reactions
Deaths caused by iodinated contrast agents are very rare, occuring at a rate of 1.1–1.2 per million contrast media packages distributed. Most are attributed to renal failure, anaphylaxis or allergic reaction.18
Almost all fatal reactions occur in the minutes following injection and patients must be under close observation during this time. The fatal event may be preceded by trivial events, such as nausea and vomiting, or may occur without warning. The majority of deaths occur in those over 50 years of age. Causes of death include cardiac arrest, pulmonary oedema, respiratory arrest, consumption coagulopathy, bronchospasm, laryngeal oedema and angioneurotic oedema.