Breast cancer screening is a recognized tool for early detection of the disease in asymptomatic women, improving treatment efficacy and reducing the mortality rate. There is raised awareness that a “one-size-fits-all” approach cannot be applied for breast cancer screening. Currently, despite specific guidelines for a minority of women who are at very high risk of breast cancer, all other women are still treated alike. This article reviews the current recommendations for breast cancer risk assessment and breast cancer screening in average-risk and higher-than-average-risk women. Also discussed are new developments and future perspectives for personalized breast cancer screening.
Key points
- •
We live in an era of risk prediction, tailored screening, and personalized treatment.
- •
It is important to be aware that some current screening recommendations might be driven by cost-effectiveness considerations.
- •
Individual screening recommendations should be made through a shared decision-making process, enabling women to make an informed decision with their physicians based on their particular risks and priorities.
- •
Recent advances in breast MR imaging, especially abbreviated protocols, support the increasing use of this method as an excellent alternative to mammography for screening women with higher than average risk.
- •
Further studies should be performed to ensure the best screening strategy for each specific population.
Introduction
Breast cancer screening is a recognized tool for early detection of the disease in asymptomatic women, improving treatment efficacy and reducing the mortality rate. Multiple historic randomized controlled trials have demonstrated the benefit of breast cancer screening using mammography. A recent Swedish trial, with a population of more than half million women, demonstrated a 41% reduction in the risk of dying from breast cancer in 10 years and a 25% reduction in the rate of advanced breast cancers, in participating women versus those not participating in the screening. Despite the consensus regarding its benefits, the screening recommendations have been widely debated over the past years and controversies remain regarding the optimal screening strategy.
Because breast cancers are heterogeneous and have their own particularities, , each woman is also different with unique risk factors, so the need for breast cancer screening varies from one woman to another. , Other imaging tools besides mammography, such as digital breast tomosynthesis (DBT), ultrasound (US), and MR imaging, play an important role in supplemental screening, particularly in women with an elevated risk of breast cancer, in whom mammography alone has a lower accuracy. ,
These understandings have raised awareness that a “one-size-fits-all” approach cannot be applied for breast cancer screening. Currently, despite the specific guidelines for a minority of women who are at very high risk of breast cancer (eg, BRCA1, BRCA2, and other known genetic mutation carriers, or patients with strong family history of breast or ovarian cancers), all other women are still treated alike.
This article reviews the current recommendations for breast cancer risk assessment and breast cancer screening in average-risk and higher-than-average-risk women, and discusses new developments and future perspectives for personalized breast cancer screening.
Breast cancer risk assessment
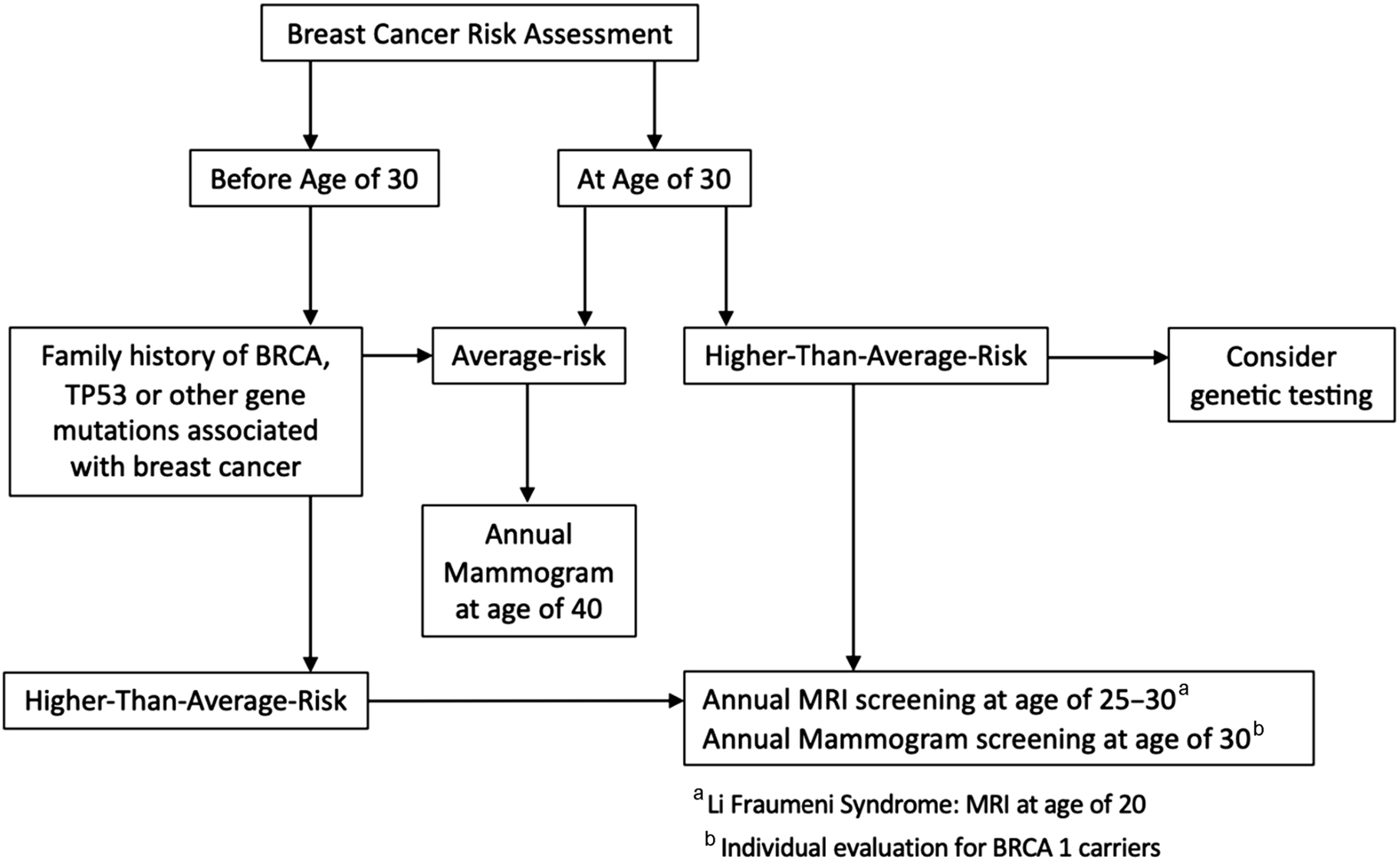
Breast cancer risk assessment should be performed at a young age because hereditary breast cancer syndromes are associated with early onset breast cancer and patients with these syndromes need tailored screening recommendations. The American College of Radiology (ACR) recommends that breast cancer risk assessment should be performed in all women at the age of 30 years to guide counseling on surveillance, genetic testing, and risk reduction treatments.
In clinical practice, risk assessment is performed with validated statistical tools to calculate the lifetime risk of breast cancer, including Gail, Claus, Tyrer–Cuzick, BRCAPRO, and BOADICEA (Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation) models, which are based on classical risk factors, such as age, family history of breast and ovarian cancer, and personal medical and reproductive history ( Table 1 ). All these models have been validated in specific populations and have limitations; thus, it is important to know which models are not applicable or possibly less accurate for a specific patient. Mathematical risk assessment models also vary in their ability to accurately incorporate risk associated with personal history of high-risk lesions, such as atypical ductal hyperplasia and lobular neoplasia, and most of these models do not include mammographic density assessment, which helps to predict individual risk of breast cancer. Recently, Yala and colleagues showed promising results from deep learning models using mammographic images demonstrating a substantially improved risk assessment compared with an established breast cancer risk model as Tyrer-Cuzick. They also demonstrated increased accuracy with a hybrid deep learning model, which used traditional risk factors and mammogram images, showing an accurate risk assessment model at an individual level, making breast cancer screening more personalized than ever.
| Model | Personal History of Breast Disease | Personal History of Ovarian Cancer | BRCA Gene | Family History of Breast and Ovarian Cancer | Ashkenazi Inheritance | Breast Density | Hormonal, Reproductive, and Other Factors |
|---|---|---|---|---|---|---|---|
| BOADICEA v5 | Breast cancer | Yes | Yes | First- and second-degree female and male relatives | Yes | Yes | Age of menarche and first live birth, menopause status, HRT, weight |
| BRCAPRO | Breast cancer | Yes | Yes | First- and second-degree female and male relatives | No | No | Ethnicity |
| Claus | Breast cancer | Yes | No | First- and second-degree female and male relatives | No | No | No |
| Gail | ADH, ALH | No | No | First-degree female relatives | No | No | Age of menarche and first live birth, ethnicity |
| Tyrer-Cuzick v8 | ADH, ALH, LCIS | Yes | Yes | First-, second-, and third-degree female relatives; first-degree male relative | Yes | Yes | Age of menarche and first live birth, menopause status, HRT, weight |
Because most available risk assessment models are not applicable to women with hereditary cancer syndromes, it is essential to determine if a patient is a candidate for genetic counseling and genetic testing before being submitted to a breast cancer risk assessment tool. There are also several available tools to guide referral for genetic counseling, including the National Comprehensive Cancer Network (NCCN) guidelines, Ontario Family History Assessment Tool, Manchester Scoring System, and Referral Screening Tool. In general, patients should be considered for genetic counseling if they have a personal or family history of ovarian cancer at any age, breast cancer at 50 years of age or younger, bilateral or triple-negative breast cancer at any age, male breast cancer, or Ashkenazi Jewish heritage. The genetic specialist then determines whether and which genetic testing is appropriate for each patient. Fig. 1 shows a breast cancer risk assessment algorithm.
There is a proliferation of genetic tests being developed for screening; however, genetic testing is still a work in progress. Most genetic testing criteria have been based on the probability of having a pathogenic variant in the BRCA1 and BRCA2 genes, which are high-penetrance genes. However, new technologies have allowed the development of multigene panel tests, which include other individual genes where variants confer a moderate to high risk of breast cancer, such as TP53 (Li-Fraumeni syndrome), PTEN (Cowden syndrome and the Bannayan–Ruvalcaba–Riley–Smith syndrome), CDH1, STK11, BRIP1, PALPB, CHEK2, and ATM.
Although only a small proportion (≤10%) of breast cancers are caused by hereditary mutations in single, dominantly acting genes, currently available evidence suggests that a larger fraction of sporadic breast cancer cases might be attributable to the action of multiple genes. In particular, polygenic risk scores based on low-penetrance single-nucleotide polymorphisms have been shown to play an important role in breast cancer risk assessment and will probably be more broadly used in the future. To date, it seems that the polygenic risk score information is actually additive to the information provided by conventional risk assessment tools. ,
Screening of average-risk women
A woman is considered to be at average-risk if she has 15% or less lifetime risk of breast cancer on risk assessment tools. The American Cancer Society (ACS) considers that an average-risk woman should not have personal history of breast cancer, strong family history of breast cancer, high-risk predisposition syndromes or genetic mutations, or history of thoracic radiation therapy before the age of 30 years. Mammography is still the mainstay of breast cancer screening because it is broadly available, with established quality assurance, and has been tested within prospective randomized trials. In addition, the ACR also states it may be appropriate to consider adding handheld or automated breast US to mammography in women with dense breasts, thereby increasing the rate of cancer detection weighed against the risk of increasing the false-positive rate significantly with screening US.
Unlike in Europe, where many countries have organized national screening programs, , the United States has opportunistic screening and many specialty societies publish divergent recommendations regarding the frequency of screening and the age range in which screening should be performed ( Table 2 ).
| ACR | NCCN | ACS | ACP, USPSTF | EUSOBI | ESMO | |
|---|---|---|---|---|---|---|
| Age to initiate (y) | 40 | 40 | 45; offer at 40–44 | 50; individualize at 40–49 | 50; consider also 40–49 | 50; consider also 40–49 |
| Screening interval | Annual | Annual | Annual for 40–54; biennial or annual >55 | Biennial | Biennial for 50–69; annual for 40–49 | Annual or biennial for 50–69 |
| Age to end | Not yet established; continue if life expectancy >5–7 y | Not yet established; continue if life expectancy ≥10 y | Continue if life expectancy ≥10 y | 74 | 69; consider also 70–74 | 69; consider also 70–74 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree