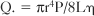Chapter 7 Ischemic Heart Disease
ISCHEMIC HEART DISEASE
Chest Radiograph
Heart Size
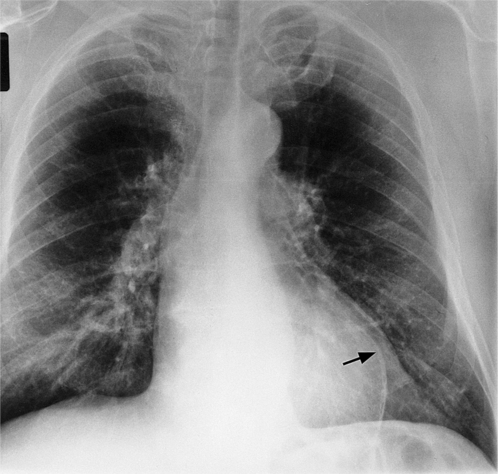
Initially, most patients undergoing myocardial infarction have a normal heart size. The assessment of an enlarged heart on a supine portable chest film is rather inaccurate and usually not necessary for clinical management. Severe cardiomegaly generally indicates long-standing coronary artery disease with previous infarction or with a major complication (Fig. 7-1).
Aneurysms and Ruptures
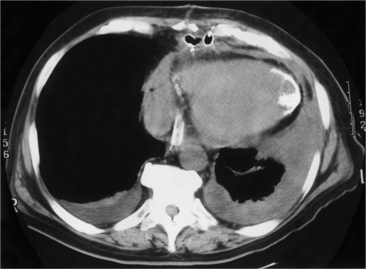
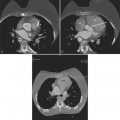
In addition to chronic left ventricular failure, left ventricular enlargement may also be the result of a true or false aneurysm, chronic mitral regurgitation, or rarely cardiac rupture. Heart enlargement is not a feature of acute mitral regurgitation or rupture of the interventricular septum because the left ventricle needs several hours to several days to dilate enough to be visible on the chest film. The most frequent site of a true left ventricular aneurysm is in the anterolateral and apical wall. Although left ventricular aneurysms may involve any wall segment, aneurysms in the posterolateral wall are frequently false aneurysms. A false left ventricular aneurysm exists when the left ventricle ruptures into a site of previous pericardial adhesions so that the rupture is contained by the pericardium. An increase in size of the left ventricular aneurysm on serial studies is suggestive of a false aneurysm and warrants urgent, definitive evaluation. Calcification of the anterolateral and apical region of the left ventricle usually takes several years after the myocardial infarction that produced the scarring (Fig. 7-2).
Coronary Angiography
Uses and Analysis
Patients with Angina Class II to IV or Unstable Angina
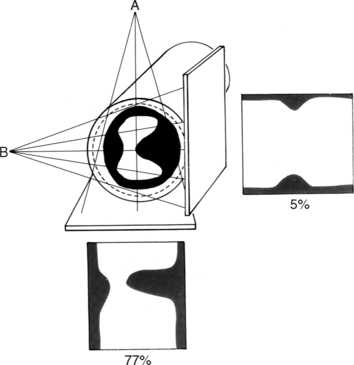
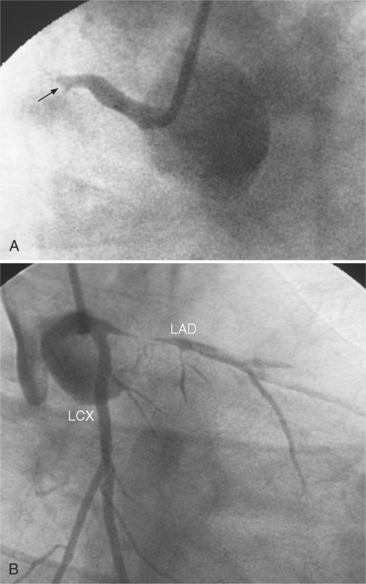
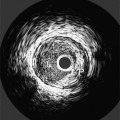
Many patients with moderate or severe stable angina or unstable angina do not respond adequately to medical therapy. PCI or CABG surgery may even improve their left ventricular systolic function. Coronary angiography is used mainly to identify atherosclerotic coronary stenosis, and less often, congenital anomalies and manifestations of other diseases. Each coronary artery is opacified so that its origin, course, and termination are visualized at least in two orthogonal projections. The orthogonal projections are necessary because atherosclerotic stenoses tend to be eccentric; a minor plaque in one projection may appear as a major stenosis in the opposite oblique view (Fig. 7-3).
Progression of Atherosclerosis
Coronary atherosclerosis begins as lipid deposition in the arterial wall, which appears grossly as a raised, fatty streak. As the lesion progresses, a fibrous cap develops over the endothelial lipid deposit. Disruption of an atherosclerotic plaque results in fissuring and intraluminal thrombosis (Fig. 7-4). The thrombus may lead to intermittent vessel occlusion and unstable angina. Large ulcers at the site of the plaque can cause formation of a fixed thrombus and a chronic occlusion resulting in acute myocardial infarction. Severe stenoses tend to progress to total occlusion about three times more frequently than less severe lesions.
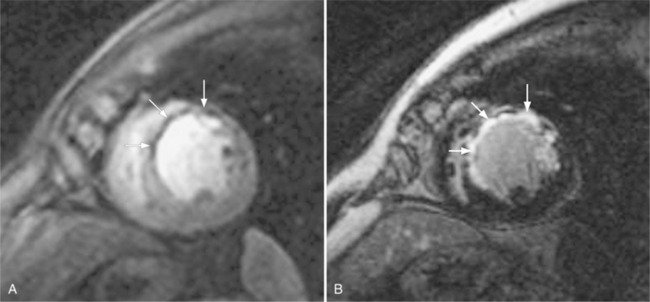
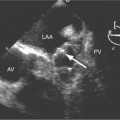
These two conditions of reversible left ventricular dysfunction are important to recognize because vigorous treatment of the thrombus or spasm in myocardial stunning and relief of the obstruction in myocardial hibernation may reverse the impaired ventricular performance and potentially salvage the jeopardized myocardium. Stress echocardiography is the modality of choice to assess left ventricular wall motion abnormalities. Stress cardiac magnetic resonance imaging (CMRI) has demonstrated better sensitivity and specificity, but is less available. Furthermore, CMRI is capable of providing useful information about left ventricular viability (Fig. 7-5).
Extent and Location of Stenoses
Common Lesion Sites
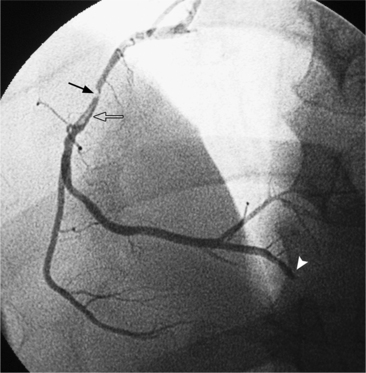
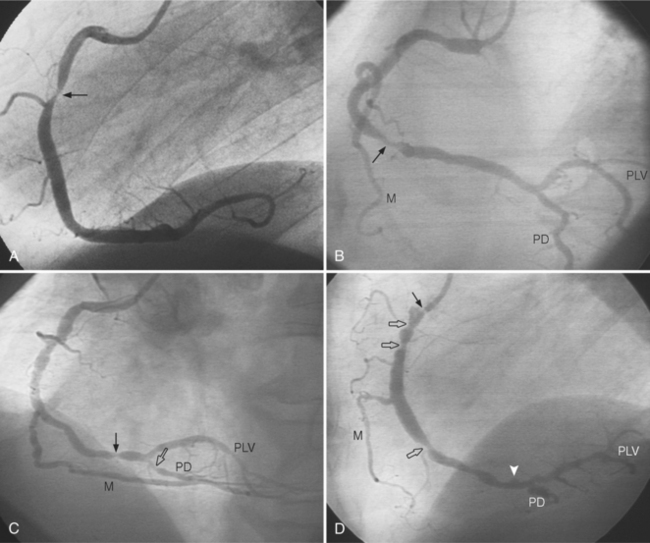
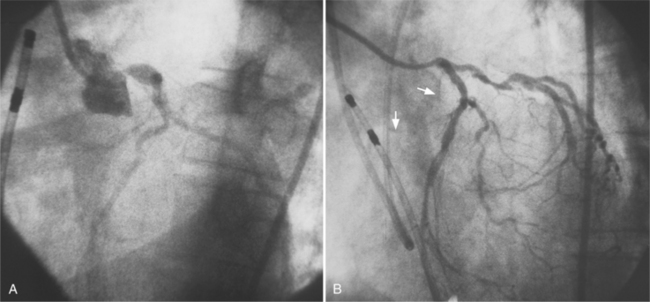
In the right coronary artery, most severe stenoses develop in its proximal half, although there are occasional severe plaques at the bifurcation of the posterior descending and posterior left ventricular arteries (Fig. 7-6). The right ventricular marginal branch frequently has a severe stenosis at its origin.
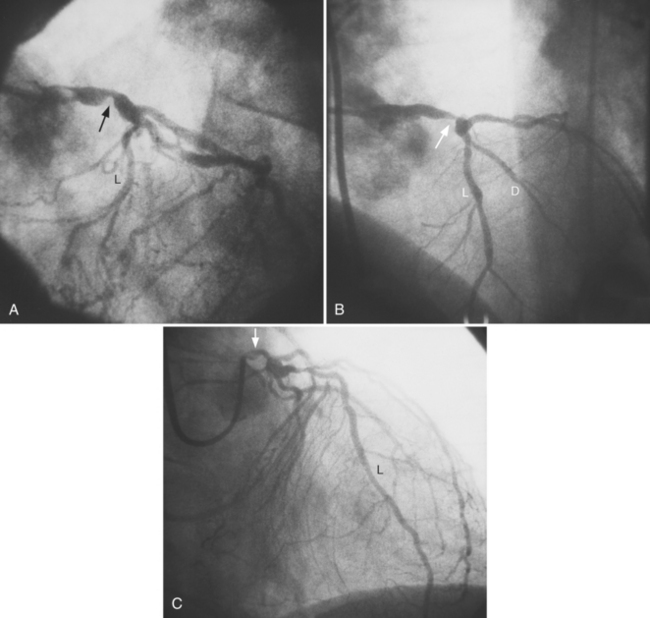
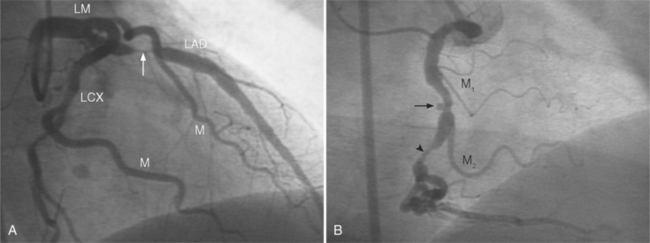
The left main coronary artery should not taper. It is usually narrowed either at its ostium or at the bifurcation of the left anterior descending and circumflex arteries. Occasionally, the entire main arterial segment may be uniformly narrowed but usually one of its ends is more severely involved. Detection of plaques in this segment is particularly important because severe lesions are associated with an increased mortality during cardiac catheterization (Fig. 7-7).
Left Main Equivalent Disease
An example of a left main equivalent lesion would be a severe left anterior descending artery stenosis when an occluded right coronary artery is supplied by collaterals from the left anterior descending artery. Here one lesion controls the blood supply to the bulk of the heart. A similar example would be a stenosis in a long left anterior descending artery that extends completely around the apex in place of the usual posterior descending artery. Here also a significant percentage of myocardium is affected by a single stenosis.
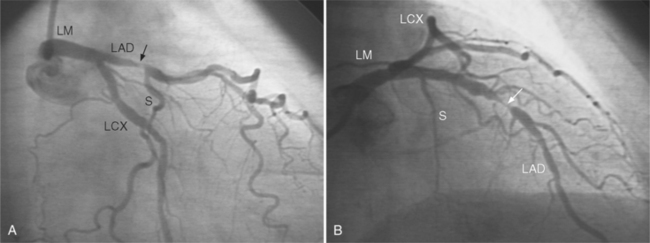
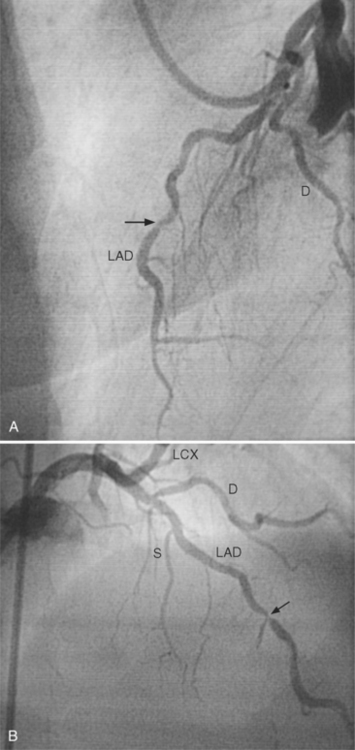
In the left anterior descending artery, stenoses before or after the first large septal branch may have different clinical implications. Patients with chronic stable angina who have a severe stenosis before the first septal branch have a statistically higher mortality when compared with patients who have a stenosis distal to this branch (Fig. 7-8). The first septal branch can supply nearly half of the interventricular septum and a contractile portion of the ventricle and is closely related to the conduction system. A stenosis before the first septal branch also frequently involves a large diagonal branch that supplies a portion of the lateral wall. This correlation does not hold in unstable angina pectoris, where there is no association between severe plaques before and after the first septal branch.
Morphology of Coronary Atherosclerosis
Angiographic Appearance
Atherosclerotic plaques tend to have eccentric and sharp edges that help distinguish them from spasm, which is usually smooth and fusiform. Thrombus within the artery may be distinguished from plaques if contrast medium flows on both sides of the lucency (Fig. 7-9). Occlusive thrombi may be impossible to distinguish from a fibrosed artery, but sharp, slanting edges are more typical of thrombus.
Grading System
Plaque morphology can be analyzed by location, shape, and severity (Box 7-1). Each lesion is resolved into length, calcification, involvement of major branches, presence of adjacent thrombus, tortuosity of the involved segment, and eccentricity of the plaque edges.
Relationship to Coronary Syndrome
Both clinical and angiographic studies have confirmed that angiographic morphology is correlated with unstable coronary syndromes. Simple plaques with a smooth fibrous covering, smooth borders, and an hourglass configuration are associated with stable angina. Complex lesions with plaque rupture, intraplaque hemorrhage, and irregular borders in eccentric stenoses are associated with unstable angina and myocardial infarction (Fig. 7-10).
Myocardial Infarctions
Multiple myocardial infarctions result from extensive coronary disease and occur with greater frequency in persons with diabetes. You are more likely to find a decreased ejection fraction in patients with diabetes mellitus but patients with diabetes and those without do not differ significantly in number and extent of severe stenosis. Type II hyperlipoproteinemia is associated with extensive coronary calcifications and severe involvement of the distal distribution of the coronary arteries (Fig. 7-11). These patients may have an unusual edge in the left sinus of Valsalva at the ostium of the left coronary artery, which can make catheterization difficult. Patients with type IV hyperlipoproteinemia have a distribution of stenoses similar to that in patients with normal lipid findings.
Other Causes of Stenosis
There are many causes of coronary stenosis other than atherosclerosis. Frequently, the cause can only be determined by clinical correlation with a systemic disease (Box 7-2). Even then, in the adult age range, it is often impossible to exclude coexisting atherosclerosis. The clinical constellation of chest pain, a positive exercise test, and a normal coronary arteriogram is referred to as syndrome X. The cause of the syndrome is unknown, is not related to large vessel spasm, and may be related to abnormalities in precapillary vessels that are too small to be seen with coronary angiography. In contrast, myocardial infarction can occur with a normal coronary arteriogram. This event is rare and has been caused by thrombosis with recanalization, coronary spasm, cocaine abuse, viral myocarditis, chest trauma, and carbon monoxide intoxication.
Interpretation of Arterial Stenoses on Angiography
Determining Severity
Because atherosclerotic plaques tend to be eccentric, coronary angiography must be performed in two orthogonal projections so the maximal arterial narrowing can be identified (Fig. 7-12). This system has many limitations. The normal-appearing artery may itself be diffusely diseased. A similar percentage of stenosis in a smaller distal artery is ascribed the same physiologic consequence, even though flow through a larger proximal arterial segment must be quite different. In a large artery with a greater cross-sectional area, the amount of myocardium supplied by its coronary flow is proportionate to the smaller area supplied by a distal coronary artery. A similar degree of narrowing of a small distal coronary artery produces the same profusion deficit as does the same percentage of stenosis in a large or proximal artery. The length of a coronary stenosis is important but is difficult to subjectively evaluate as to severity of a lesion reducing distal flow. Given these limitations, a 50% or greater stenosis in a patient with ischemic heart disease is defined as a significant stenosis.
Determinants of Coronary Blood Flow
Normal Flow
where Q. is flow per unit time, r is radius, P is pressure, L is length, and μ is viscosity. This equation is strictly valued for nonpulsatile, streamline flow and a uniform viscosity. With some allowance for the transfer of this mathematical principle to a biologic system, the equation helps explain some of the determinants of coronary flow. Under normal conditions, all the variables in the equation are constant except for the radius of the vessel. However, a number of factors act on the major site of vascular resistance: the precapillary arteriole.
Flow Reserve
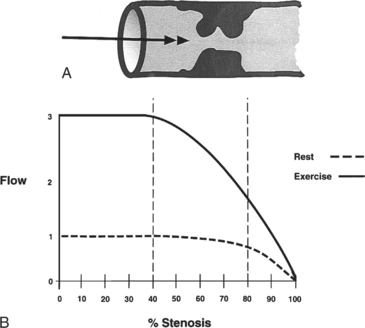
Coronary flow reserve is the maximal flow divided by the resting flow. The “50% significant stenosis” is then a rough approximation to this physiologic model. The maximal flow is that which occurs when the coronary vascular bed has undergone maximal vasodilatation. Fig. 7-13 shows the relation between coronary blood flow and a focal stenosis in an artery at rest and after maximal vasodilatation.
Fractional Flow Reserve
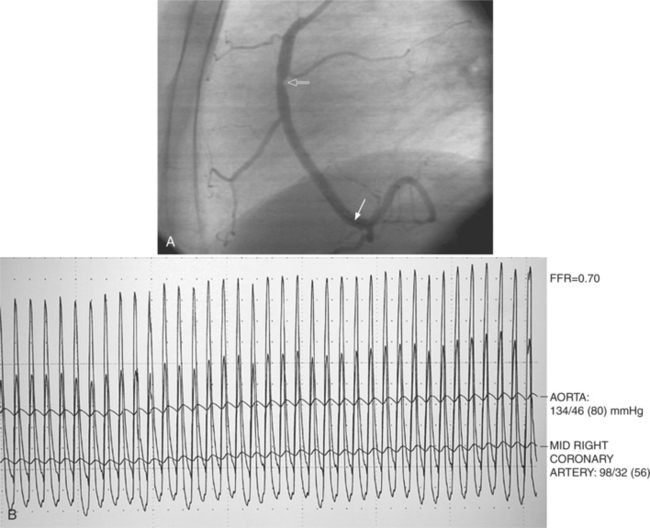
Fractional flow reserve (FFR) is an invasive index of the functional severity of a stenosis determined from coronary pressure measurement during coronary catheterization. A 0.014-in pressure wire is advanced, after calibration, into the relevant coronary segment. It is positioned distally to the stenosis. Adenosine is administered intravenously to induce maximal hyperemia. FFR is calculated as the ratio of mean hyperemic distal coronary pressure measured by the pressure wire to mean aortic pressure measured through the guiding catheter. In patients with coronary stenosis of moderate severity, FFR appears to be a useful index of the functional severity of the stenosis and the need for coronary revascularization. An FFR value of less than 0.75 demonstrates a reversible myocardial ischemia (Fig. 7-14) with a sensitivity of about 90% and a specificity of 100%.
Effects of Stenosis Length and Diameter
The length of the coronary stenosis also determines blood flow, although its effect is complex. When the diameter is constricted by less than 50%, stenoses of up to 15 mm in length have little effect on flow during reactive hyperemia. As the diameter of a stenosis is increased to 70%, a stenosis 10 mm long results in a reduction in distal flow. Long stenoses in the presence of borderline reduction in diameter (40% to 70%) may greatly alter coronary hemodynamics during stress. Although Poiseuille’s equation suggests that the length of a stenosis would decrease flow linearly, this cannot be precisely observed because there are neural and biochemical factors and collateral flow that also regulate blood flow. Moreover, the distal coronary vascular bed may vasodilate in a nonuniform manner from endocardium to epicardium. Because a proximal stenosis reduces coronary pressure, the endocardium becomes anoxic before the epicardium does, and there is increased lactate production and a fall in highenergy phosphate mediators. The end result is that vascular resistance distal to a stenosis in an ischemic myocardium varies in a complex way.