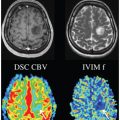
IVIM MRI of the Placenta
16.1 Introduction
The placenta enables the transfer of oxygen and nutrients to the growing fetus and is vital to the lifelong health of both mother and child [1, 2]. It is highly vascular, contains large volumes of maternal and fetal blood, and undergoes major structural and functional changes across the 40 weeks of gestation. It therefore presents unique challenges and opportunities for intravoxel incoherent motion magnetic resonance imagery (IVIM MRI).
16.1.1 Placental Markers of Pregnancy Complications
Many major pregnancy complications are associated with placental malfunction [3]. Deviations in blood flow can disrupt the transfer of oxygen and nutrients to the developing fetus, eventually leading to complications such as fetal growth restriction [4–6] (FGR, defined as growth below the genetic potential of the fetus) and pre- eclampsia [7, 8] (PE, a disease characterized by elevated maternal blood pressure and proteinuria). Despite these underlying placental etiologies, both FGR and PE are difficult to detect before the onset of symptoms. In other words, at the point of FGR or PE diagnosis there has already been substantial inhibition of placental function. Biomarkers that quantify placental development—with their potential for prediction, earlier diagnosis, and better management of high-risk pregnancies—are therefore of great importance.
16.1.2 Imaging Markers of Pregnancy Complications
Ultrasound is currently the main modality for pregnancy monitoring. A wide range of ultrasound-derived biomarkers are currently used in the clinic, such as fetal biometry, heart rate, amniotic fluid volume, and Doppler ultrasound of the umbilical cord and uterine arteries. FGR is generally diagnosed by a combination of Doppler ultrasound of the umbilical cord and uterine arteries and fetal biometry abnormalities [9–11]. However, there are many limitations to placental ultrasound: it has a small field of view limiting whole- organ assessment, especially later in gestation; low contrast; and provides limited functional information [12].
16.1.3 Challenges and Unique Potential of Placental MRI
MRI can probe both structure (what tissue is present and its configuration) and function (e.g., blood flow, oxygenation, and nutrient exchange). It therefore offers a unique perspective on the placenta and has the potential to address the limitations of ultrasound. MRI has been recently shown to offer improvements in sensitivity and specificity; for example, mean placental T2* is a stronger predictor of low birth weight than Doppler ultrasound [13]. However, there are a number of factors that limit the utility of placental MRI. These include maternal and fetal motion, uterine contractions, and magnetic field inhomogeneity. It is likely that the uniqueness of the placenta means that bespoke solutions to these problems are required. Recent work has made significant progress toward these goals [14–17], hence increasing the clinical viability of placenta MRI.
16.1.4 Diffusion MRI of the Placenta
In particular, diffusion MRI (dMRI)—withits potential for noninvasive assessment of tissue microstructure and microcirculation—is emerging as a promising tool for placental assessment. There are a small, but growing, number of in vivo human placenta dMRI studies in the literature. A majority of these studies have utilized IVIM MRI (we review these later in the chapter). In addition, placenta dMRI studies have been performed using apparent diffusion coefficient (ADC) [18–21] and diffusion tensor imaging (DTI) [22] models.
16.1.5 Placenta Structure
The placenta gets attached to the maternal uterine wall and connected to the fetus via the umbilical cord. It is typically around 20 cm across and 2 cm thick and consists of 30–40 lobules (also known as cotyledons). Each lobule contains one or two functional units containing a paired maternal spiral artery and fetal villous tree. Figure 16.1 is a schematic of the placenta structure and outlines the principal blood flow routes. A fetal villous tree consists of a large stem villous attached to the chorionic plate; this stem subsequently branches into a network of smaller intermediate villi, which further divide into a network of capillarized terminal villi. Maternal spiral arteries and decidual veins are located immediately adjacent to the placenta in the uterine wall, which consists of various fibrous cell types and trophoblastic cells.
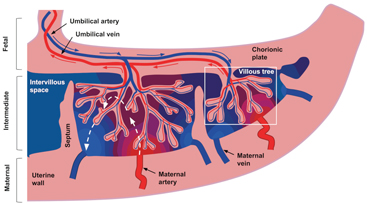
Figure 16.1 Schematic representation of blood flow through the placenta and surrounding tissue. The blue and red arrows show the flow directions of oxygenated (red) and deoxygenated (blue) fetal blood through the placental vasculature. For clarity, only the largest villi are included. The dashed white arrows show idealized flow lines through the intervillous space for maternal blood. Idealized oxygenation states are represented by the color gradient from red to blue. Figure and caption from Ref. [23], used under the CC BY license.
16.1.5.1 Movement of blood
There are two nonmixing blood types in the placenta—fetal and maternal—both of which have very different flow characteristics. Fetal blood resides in the fetal vasculature and perfuses at high pressure through the convoluted villous network. On the other hand, and quite uniquely, maternal blood doesn’t flow within vasculature. Instead, it flows at low pressure through spiral arteries into the intervillous space, submerging the fetal villous trees and thereby facilitating oxygen exchange with fetal blood across the villous tree surface.
16.1.5.2 Development across gestational age
The placenta undergoes major functional and structural changes across the 40 weeks of gestation in order to support the developing fetus. For example terminal villi—the principal location of oxygen exchange—develop mainly after 20 weeks [24]. Additionally, calcification generally begins from Week 37 onward, and premature onset has been linked to adverse maternal and fetal outcomes [25–27]. These changes offer both challenges and unique opportunities. Quantitative methods need to be sensitive to the full range that parameters can take over gestation. If such methods could accurately characterize these physiological and pathological developments this would be of great interest clinically.
IVIM MRI provides a unique tool for working toward this goal.
16.1.5.3 How the placenta structure affects the dMRI signal
Due to the unique circulatory structures, it is not immediately clear how the placental dMRI signal will be attenuated at different b values. Our thoughts on how placental structure, microstructure, and microcirculation may affect the dMRI signal are as follows. We expect that blood flowing incoherently within fetal vasculature exhibits a pseudodiffusive effect at the voxel scale and hence contributes a fast-attenuating dMRI signal component. We also expect a slow- attenuating dMRI signal component due to water in tissue, such as fetal villous tree walls. It is far from clear how maternal blood flow will impact the dMRI signal. It may exhibit fast incoherent flow on the voxel scale when within vasculature, for example, spiral arteries and decidual veins. Therefore, a fast-attenuating component to the dMRI signal is likely within the uterine wall. However, once it has entered intervillous space, its behavior is very different. Any coherent flow of maternal blood should cause little signal attenuation. However, diffusion within this flow should cause some signal attenuation— with a diffusivity comparable to water at body temperature (3 ¥ 10–2 mm2 s–1). Additionally, percolation of maternal blood through highly convoluted spaces proximal to fetal villi (in order to facilitate oxygen exchange) may appear incoherent at the voxel scale. If this is the case, it is very difficult to know the speed at which this blood would be percolating and hence what dMRI signal component (fast attenuating, slow attenuating, or otherwise) this would affect. Previous work in mouse placentas by Solomon et al. sheds some light on the problem [28]. They found that maternal blood had near-free diffusion behavior, whereas fetal blood flows 2 orders of magnitude quicker. However, whether this observation translates to human placentas remains unclear.
16.2 IVIM in the Placenta
The first in vivo measurements of IVIM MRI parameter values (in fact, the first applications of dMRI) in the human placenta were presented in two publications in 2000 [29, 30]. The first [29] presents IVIM results from 11 healthy pregnant subjects to give an idea of the ranges and trends over gestation of all three IVIM parameters: the perfusion fraction, pseudodiffusivity, and diffusivity. FGR cases were scanned in the second article, and an IVIM-derived parameter was identified—the difference in perfusion fraction values between outer and inner placental regions of interest (ROIs)—that was lower in FGR pregnancies [30].
In subsequent placental studies, IVIM has remained the most common model for interpreting dMRI data. These papers have generally followed one of the approaches in these first two papers: try to characterize the trajectory of IVIM parameters across gestational age, or try to identify an IVIM parameter that is significantly altered due to pathology. Table 16.1 gives a summary of the published studies of IVIM MRI applied to the in vivo human placenta.
Table 16.1 Reported diffusion MRI parameters in the placenta for in vivo human pregnancies
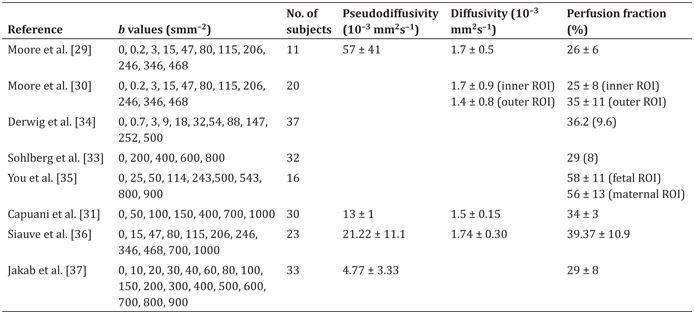
Note: Values are reported as either mean ± SD, or median (IQR). All studies were performed at 1.5 T, excluding both studies by Moore et al. (0.5 T) and some of the subjects in Jakab et al. (3 T). Each study includes a variety of subjects, either with or without disease; we refer the reader to the original studies for full details. Capuani et al. [31] did not report parameter values in the text; the values we present were read from the figures. We have not included a second paper by Sohlberg et al. [32] as it contains the same subjects as those in Ref. [33].
16.2.1 Trends across the Gestational Age
There are two main reasons for characterizing biomarker trends across gestation. Since many change significantly throughout normal gestational aging, knowledge of typical biomarker trends is a requirement to identify deviation. Additionally, we know that the placenta undergoes dramatic microstructural changes across gestation. If we can identify the dMRI-derived parameters that best capture these changes, it follows that these will be good candidates for detecting pathological microstructural changes.
There is variability in the reported trends for IVIM MRI parameters across gestational age. Three studies, all of which began scanning at 22 weeks, have shown a clear decrease in perfusion fraction across gestation [29,33,35]. Siauve et al. scanned at earlier gestations (starting at 16 weeks) and found an increase in perfusion fraction between 16 and 22 weeks of GA, stability between 22 and 28 weeks, and then a decrease [36]. On the other hand, two recent papers have shown an apparent increase in perfusion fraction across gestation [31, 37].
There are a number of factors that could cause this interstudy variability. These include the lack of a common protocol, variability in ROI selection, different gestational age (GA) ranges, and variation in the types of pregnancy included. For example, Capuani et al. used small ROIs within the placenta, rather than a whole-organ average, to calculate perfusion fraction values. Compared to other studies, Jakab et al. have a much larger range in perfusion fraction values, which may reflect differences in the dMRI protocol or model fitting procedure. Clearly further studies are necessary in order to account for these discrepancies. How these factors specifically affect the observed trend in IVIM perfusion fraction across gestation is an important area for future work and of crucial importance for any future use of the perfusion fraction as a biomarker.
16.2.2 Parameter Variation with Pathology
The ultimate aim of IVIM MRI in the placenta is to identify param- eters, or combinations thereof, that are sensitive to the presence or severity of pathology. Thus far, although we should emphasize the small number of studies, IVIM-derived parameters have shown reasonable discrimination between normal and pathological placen- tas. For example, the perfusion fraction appears significantly lower in placentas associated with FGR [30,32,34] and early onset PE [33]. Additionally two studies show that ADC, which averages over IVIM effects, was decreased in FGR [19, 21]. However, it should be noted that the choice of b values heavily affects the inferred ADC val- ues [18].
16.3 Anisotropic IVIM in the Placenta
While standard models for analyzing dMRI data, such as IVIM, ADC, and DTI, have shown promise in the placenta, it has been shown in other tissues that advanced microstructural models can extract more specific biophysically linked parameters from the signal. We recently undertook a study to assess which microstructural models best explain dMRI data in the placenta [23].
16.3.1 dMRI Scans
We scanned 9 healthy pregnant subjects with a rich, multishell, multidirectional dMRI protocol. The three principal gradient directions were scanned at b = 15, 25, 80, 115, 206, 246, and 346 s mm–2; eight directions were obtained at b = 40, 400, 1000, and 2000 s mm–2; and there were six b = 0 images. We used this acquisition, which is much richer than would typically be available, in order to assess the most expressive models that the placental dMRI signal potentially supports.
16.3.2 Modeling
The models we fit to the data were motivated by our predictions of how placental structure and microstructure will attenuate the dMRI signal (i.e., Section 16.1.5.2). We chose a set of 14 microstructural models, the majority of which consist of two compartments separately modeling the fast-attenuating (primarily associated with perfusion) and slow-attenuating (primarily associated with diffusion) signal components.
In “standard” IVIM MRI, perfusion and diffusion compartments are both considered to be isotropic. This may not hold in the placenta, for example, in areas with fibrous cells or where vasculature has a coherent orientation. We therefore included models that allow anisotropy in perfusion and diffusion compartments and can therefore be considered anisotropic extensions to IVIM. We found it informative to categorize our models depending on the anisotropy of these two compartments. For example, “anisotropic–isotropic” refers to models with anisotropic perfusion compartment and isotropic diffusion compartment.
All models were combinations of compartments as described in Ref. [38], such as ball, stick, zeppelin, and tensor. “Ball” represents an isotropic diffusion compartment (i.e., an ADC model). A “tensor” compartment models the signal using a full diffusion tensor, and “zeppelin” is a cylindrically symmetric tensor. A “stick” is maximally anisotropic, assuming that water only diffuses in a single direction. We built models using combinations of these compartments, again following the terminology in Ref. [38]; for example, the standard IVIM model is referred to as “ball–ball.”
An example of an anisotropic IVIM model is stick–zeppelin, a two-compartment model that contains anisotropic perfusion and diffusion compartments. The signal is given by
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree