and Arumugam Rajesh2
(1)
Department of Radiology, Harvard Medical School Massachusetts General Hospital, Boston, Massachusetts, USA
(2)
Department of Radiology, University Hospitals of Leicester NHS Tr Leicester General Hospital, Leicester, UK
Case 2.1
Brief Case Summary:
37 year old female with persistent, asymptomatic microscopic hematuria.
Imaging Findings
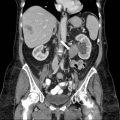
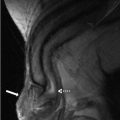
CT scan of the abdomen and pelvis with intravenous and oral contrast demonstrates the absence of a kidney in the expected location of the left renal fossa. A portion of the right kidney is noted (arrow in Fig. 2.1a). Note the flattened appearance of the left adrenal gland (the “lying down”) compatible with a congenitally absent left renal fossa. Figure 2.1b demonstrates a displaced left kidney across the midline which is fused with a caudally located right kidney. Figure 2.1c (obtained during a delayed portion of this exam to opacify the collecting system) demonstrates the normal entry zone of both ureters (arrows).
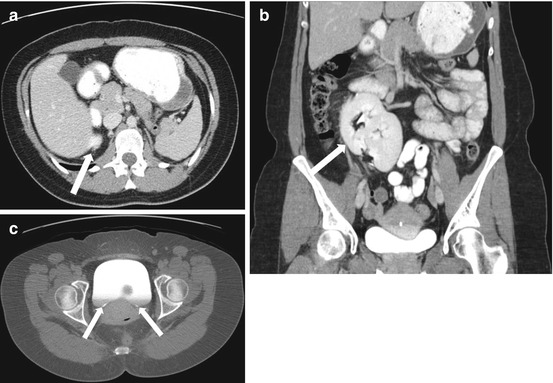

Fig. 2.1
CT scan of the abdomen and pelvis with intravenous and oral contrast demonstrates the absence of a kidney in the expected location of the left renal fossa. A segment of the right kidney is noted (arrow in a). Note the flattened appearance of the left adrenal gland (the “lying down”) compatible with a congenitally absent left renal fossa. (b) Demonstrates a displaced left kidney across the midline which is fused with a caudally located right kidney (arrow). (c) Obtained during a delayed portion of this exam to opacify the collecting system, demonstrates the normal location of both ureters (arrows)
Differential Diagnosis
Horseshoe kidney
Diagnosis and Discussion:
Crossed Fused Ectopia
Crossed fused renal ectopia is the second most common congenital renal fusion anomaly where both kidneys are on one side of the body and the renal parenchyma fuses. Patient with cross fused renal ectopic are usually asymptomatic and it is usually incidentally diagnosed. However, if symptoms are present, they are usually abdominal and flank pain, hematuria, or urinary tract infection.
Four types of crossed renal ectopia have been described according to the McDonald and McClellan classification. The most common accounts for 85 % of cases and is crossed renal ectopia with fusion. Crossed renal ectopia without fusion accounts for 10 % and solitary crossed renal ectopia and bilaterally crossed renal ectopia account for the minority of crossed renal ectopia types. The most common form is where the upper pole of the inferiorly positioned kidney fuses with the lower pole of the superiorly positioned kidney. The ureter of the ectopic kidney crosses midline and enters the bladder in the anatomic position. Most commonly, the left kidney is ectopic and is abnormally located in the right hemiabdomen.
On imaging, CT and MR are the best imaging modalities for diagnosis and classification of the type of crossed renal ectopia. CT and MR can evaluate the blood supply to the kidneys and evaluate for potential urologic complications. The blood supply to the normally-located kidney as well as the ectopic kidney commonly have aberrant vascular anatomy that can be readily evaluated on cross sectional imaging. The ectopic kidney frequently receives some blood supply from the ipsilateral vessels.
Potential complications include urinary reflux, ureteropelvic junction obstruction, and ectopic ureteroceles. The abnormal position of the kidneys predisposes to urinary stasis resulting in hydronephrosis, calculi formation, and recurrent urinary tract infections. In the pediatric population, crossed fused renal ectopia may be associated with other congenital abnormalities involving the cardiovascular, gastrointestinal, and skeletal systems.
Pitfalls
Differentiating between crossed fused renal ectopia and horseshoe kidney can be readily done by evaluating whether the kidneys are both on one side of the body in crossed fused renal ectopia or on both sides of the body as in horseshoe kidney.
Teaching Point
1.
Crossed fused renal ectopia is the second most common congenital renal fusion anomaly, second to horseshoe kidney.
2.
Congenital renal fusion anomalies are usually associated with variant renal anatomy and can be associated with other congenital anomalies.
3.
The diagnosis, variant arterial anatomy, and complications can be readily assessed with CT and MR.
Case 2.2
Brief Case Summary:
57 year old male with abdominal pain.
Imaging Findings
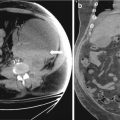
CT scan of the abdomen and pelvis with intravenous and oral contrast demonstrate midline fusion of the lower poles of the kidneys (Fig. 2.2a). Note that the fusion is located below the level of the inferior mesenteric artery as demonstrated on the 3D rendered reconstruction (arrow in Fig. 2.2b). US (Fig. 2.3) demonstrates the sonographic appearance of a horseshoe kidney in a different patient, with the fused segment lying anterior to the abdominal aorta (arrow). Non-contrast CT scan of the abdomen in the same patient as Fig. 2.3 demonstrates the presence of a non-obstructing stone within the lower pole of the left kidney (arrow in Fig. 2.4).
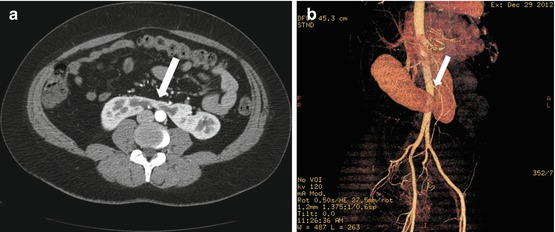
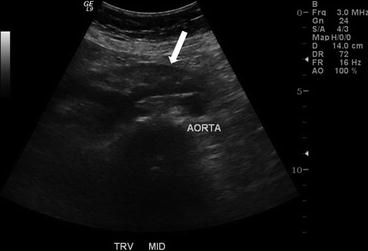
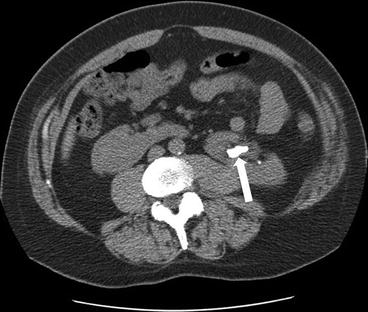

Fig. 2.2
CT scan of the abdomen and pelvis with intravenous and oral contrast demonstrate midline fusion of the lower poles of the kidneys (a). Note that the fusion is located below the level of the inferior mesenteric artery as demonstrated on the 3D rendered reconstruction (arrow in b)

Fig. 2.3
US demonstrates the sonographic appearance of a horseshoe kidney in a different patient, with the fused segment lying anterior to the abdominal aorta (arrow)

Fig. 2.4
Non-contrast CT scan of the abdomen in the same patient as Fig. 2.3 demonstrates the presence of a non-obstructing stone within the lower pole of the left kidney (arrow)
Differential Diagnosis
On ultrasound, crossed fused renal ectopia may have a similar appearance. Otherwise, no differential diagnosis.
Diagnosis and Discussion:
Horseshoe kidney
Horseshoe kidneys are the most common renal fusion anomaly with an incidence of 1 in 400 births, with males being twice as commonly affected. Patients may be asymptomatic, however, vague abdominal pain with possible radiation to the back is a possible symptom. On physical examination, a palpable abdominal mass in the midline may be noted.
The best imaging diagnostic feature is two kidneys located in the renal fossa on opposite sides of the body with fusion of the lower poles forming an isthmus of tissue crossing the midline. The isthmus crosses anterior to the abdominal aorta. The isthmus in a horseshoe kidney acts as an impediment to normal rotation and upward ascent where the isthmus encounters the inferior mesenteric artery. 90 % of horseshoe kidneys have midline or symmetrical fusion, whereas the remaining 10 % of cases have a lateral or asymmetrical fusion.
On radiography, the kidneys are close to the spine with the lower poles being the medial most portions of the kidneys. CT examination allows for assessment of the degree and type of fusion, evaluation of renal parenchymal and collecting system changes, delineation of variant renal arterial supply, and diagnosing complications. The benefit of CT includes the ability to perform multiplanar reformats and 3-D reconstructions. MR may also be performed to show fusion of the lower poles of the kidneys which crosses midline and the low position of the kidneys which are located caudal to the inferior mesenteric artery.
Other congenital anomalies that are associated with horseshoe kidneys include vertebral, esophageal, anorectal, and tracheal malformations as well as chromosomal and hematologic abnormalities.
Complications of horseshoe kidneys are not uncommon and include traumatic injury, ureteropelvic junction obstruction, recurrent infections, calculi formation, and increased risk of renal cell carcinoma. The isthmus’s location immediately anterior to the abdominal aorta and vertebral bodies predisposes the horseshoe kidney to traumatic injury, particularly due to the absence of protection by the overlying ribs. The abnormal position of the kidneys may lead to poor outflow and urinary stasis which predisposes to hydronephrosis, recurrent infection, and calculi formation.
Pitfalls
The main differential consideration for a horseshoe kidney is renal ectopia where the kidneys are congenitally located in an abnormal position. In crossed fused ectopia, the kidneys are located on the same side of the body and typically the ureter crosses midline to insert into the bladder in the normal anatomic position.
Teaching Point
1.
Horseshoe kidney is the most common renal fusion anomaly where the kidneys remain in anatomic location with fusion of the lower poles across midline forming an isthmus, located anterior to the abdominal aorta and vertebral bodies.
2.
Anomalous renal vasculature and complications including predisposition to traumatic injury, calculi formation, and recurrent infections are important associations of horseshoe kidneys.
3.
CT allows for evaluation of the degree and type of fusion in addition to associated complications, particularly with the multiplanar reformats and 3D rendered reconstruction.
Case 2.3
Brief Case Summary:
40 years old male, asymptomatic
Imaging Findings
Coronal reconstructed T1-weighted MIP MRI image shows enlarged calyces (white arrows) with normal size renal pelvis of left kidney (Fig. 2.5). Also note associated megaureter (arrow head).
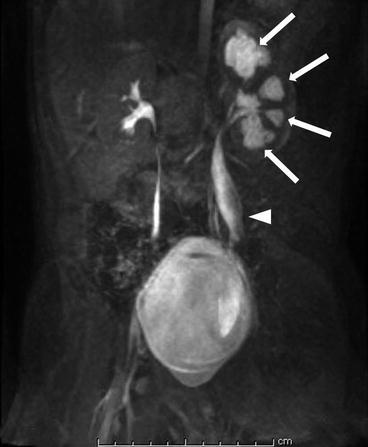

Fig. 2.5
Coronal reformatted T1-weighted MIP MRI image shows enlarged calyces (white arrows) with normal size renal pelvis of left kidney. Also note associated megaureter (arrow head)
Differential Diagnosis
Hydronephrosis, Polycalycosis, Intermittent hydronephrosis and Infundibular stenosis
Diagnosis and Discussion
Congenital megacalycosis
Congenital megacalycosis (CM), also called megacalyces or Puigvert’s disease, is a rare renal disorder first reported in 1963, which presence caliceal dilatation and hypoplasia of the pyramids of Malpighi without evidences of renal pelvic or ureteral obstruction and impaired renal function. This disease commonly involves unilateral kidney and occurs in male. This abnormality may be caused by a transient delay in the recanalization of the upper ureter after the ureteral bud connects to the metanephros or the underdevelopment of the pyramids with lack of projection of the papillae into the calyces. The calyces commonly increase to 12–20 in number and have a rounded appearance. The pyramids have a semilunar configuration, instead of the normal triangular or cone shape, and the tip of each papilla is flat. The disease is usually diagnosed by accident or by its complications such as renal calculi and urinary tract infections. It also can accompany with other urogenital malformations, such as mega-ureter or multicystic dysplastic kidney, or with other congenital disorders, such as Schinzel–Giedion syndrome.
The imaging diagnosis of CM is first established by ultrasound and intravenous urography (IVU). CM may appear as an enlarged kidney with caliectasis. IVU can display not only the morphological abnormalities, including increased renal size, decreased thickness of the parenchyma and ectatic, polygonal and outnumbered calyces, but also the functional change as delayed enhancement of the pelvicaliceal system. Both pelvises and ureters appear normal and obstructive signs are absent. This point is important when distinguishing with obstructive hydronephrosis. CT urography (CTU) and MR urography (MRU) can further demonstrate these findings, moreover, providing more details on shapes of calyces and pyramids and associated complications. Micturating cystourethrography and ascending urography excluded vesicoureteral reflux or obstruction. Scintigraphies with 99 m-dimercaptosuccinic acid (Tc99mDMSA) and Technetium 99 m-diethylenetriaminepentaacetic acid (Tc99mDTPA) usually present normal.
Pitfalls
Megacalycosis should be carefully discriminated with obstructed hydronephrosis because the former does not cause the renal function damage and thus no surgical intervention need. CM can be differentiated from obstructive hydronephrosis by imaging methods, based on the normal-sized pelvis and ureter on IVU, CTU and MRU, normal excretory function on IVU, normal display on scintigraphies with Tc99mDMSA and Tc99mDTPA. However, sometimes the findings of CM on these imaging methods also can be abnormal, which adds a little confusion on differential diagnosis. For example, CM may show a delay in visualization of the collecting system due to the large volume of the calyces. The results of scintigraphies may be abnormal due to the accompanying urinary tract infections. Renal function and radiographic appearances have remained stable in cases followed over several years.
Teaching Point
1.
The increased number and dilatation of calyces with undilated pelvis and ureter and normal renal function are the key points to distinguishing CM from obstructive hydronephrosis.
2.
IVU is the preferred diagnostic tool for CM for it combines the morphological and functional features.
3.
The complications of CM, such as urinary tract infections and renal calculi, sometimes may alter the imaging manifestations and influence the diagnosis.
Case 2.4
Brief Case Summary
None. Middle aged patient complaining of non specific abdominal pain
Imaging Findings
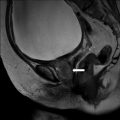
Axial contrast-enhanced CT scans demonstrate a simple renal cyst at right renal sinus displacing contrast-filled right renal pelvis (arrows in Fig. 2.6a, b).
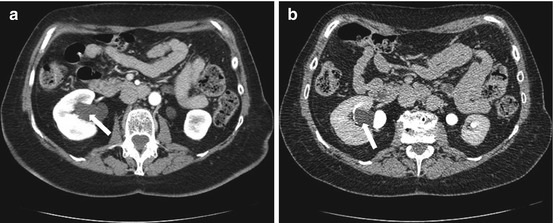

Fig. 2.6
Axial contrast-enhanced CT scans demonstrate a parapelvic cyst in the right renal sinus displacing contrast-filled right renal pelvis (arrows in a, b)
Differential Diagnosis
Hydronephrosis
Diagnosis and Discussion:
Parapelvic Cysts
Parapelvic renal cysts are lymphocytic in origin or may develop from embryologic rests and do not communicate with the collecting system. Parapelvic cysts are clinically important as they can be confused with hydronephrosis or an extrarenal pelvis. Parapelvic cysts may also compress the collecting system resulting in hypertension, hematuria, or can become secondarily infected.
On CT, parapelvic cysts are thin walled with a homogeneous appearance and attenuation of water. The parapelvic cysts are smoothly marginated and often have lobulated margins. A parapelvic cyst can also mimic a dilated renal pelvis or an extra renal pelvis on CT. After injection of intravenous contrast and imaging in an excretory phase of contrast, the normal collecting system partially or completely opacifies with contrast material and in contradistinction, a parapelvic cyst will remain unchanged in the excretory phase.
Treatment is usually not needed unless compression of the renal collecting system causing symptoms is present.
Pitfalls
Differentiating parapelvic cysts from a dilated renal pelvis, hydronephrosis, and extrarenal pelvis is clinically important as a misdiagnosis may lead to additional imaging studies or subsequent treatment, particularly for treatment of hydronephrosis.
Teaching Point
1.
Parapelvic cysts are not true cysts and are likely lymphatic in origin or may result from embryologic rests.
2.
There is no communication with the collecting system which allows for accurate differentiation from hydronephrosis on an excretory or urographic phase of a contrast enhanced CT.
3.
Accurate differentiation of parapelvic cysts from hydronephrosis will prevent additional workup and treatment.
Case 2.5
Brief Case Summary:
64 year–old male with end–stage renal disease
Imaging Findings
Non contrast CT image (Fig. 2.7a) of the patient 1 year prior shows atrophic kidneys and ascites from renal failure. Contrast enhanced current axial CT image (Fig. 2.7b) shows bilateral renal cysts of varying sizes with non obstructing left renal pelvic stone and ascites. Coronal T2-weighted image (Fig. 2.7c) shows the bilateral cysts within kidneys along with ascites.
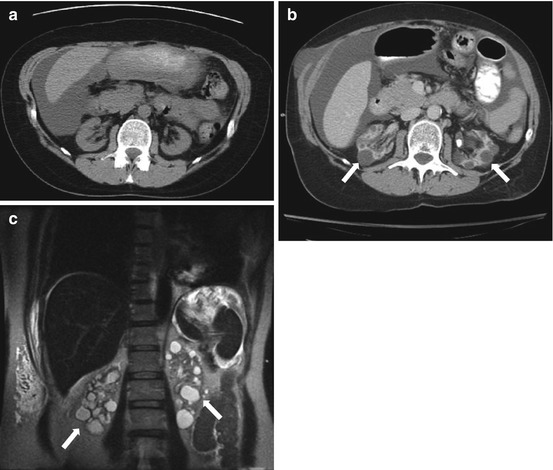

Fig. 2.7
Non contrast CT image (a) of the patient 1 year prior shows atrophic kidneys and ascites from renal failure. Contrast enhanced current axial CT image (b) shows bilateral renal cysts of varying sizes with non-obstructing left renal pelvic stone and ascites. Coronal T2-weighted image (c) shows the bilateral cysts within kidneys along with ascites
Differential Diagnosis
Multiple simple renal cysts
Tuberous sclerosis
Medullary cystic disease
Von Hippel-Lindau disease
Diagnosis and Discussion
Acquired cystic kidney disease
Patients with end-stage renal disease undergo a proliferative process in their kidneys and are at increased risk for renal cyst formation and malignancy. Acquired cystic kidney disease (ACKD) develops in patients with end-stage renal disease (ESRD), particularly in patients receiving dialysis, where multiple bilateral renal cysts develop progressively over time. The number, size, and prevalence of cysts increase with the duration of dialysis with nearly 100 % of ESRD patients treated for 10 or more years. ACKD occurs in patients treated with peritoneal dialysis and hemodialysis.
On imaging, multiple, small <1 cm, fluid-filled renal cysts are seen in atrophic to normal-sized kidneys. The cysts involve predominantly the renal cortex but may also involve the medulla. On US, multiple anechoic cysts are visualized with small to normal-sized kidneys with increased parenchymal echogenicity and loss of the corticomedullary differentiation. Hemorrhagic cysts appear as hypoechoic lesions with internal echoes with increased posterior acoustic enhancement. On CT, bilateral cysts are seen and hemorrhagic cysts are hyperdense, making accurate differentiation from malignancy difficult. MR allows for improved soft tissue characterization with <1 cm simple cysts manifesting as nonenhancing T1 hypointense, T2 hyperintense lesions. Complicated cysts have variable T1 and T2 signal depending on the proteinaceous and hemorrhagic contents. MR is helpful in assessing for soft tissue enhancement which accurately differentiates enhancing renal cell carcinoma from complicated cysts, particularly with the use of subtraction imaging.
Hemorrhage within cysts, perinephric hemorrhage, and development of renal cell carcinoma are important potential complications of ACKD. Risk factors for developing renal cell carcinoma in patients on dialysis include male sex, prolonged dialysis, and ACKD. Other potential complications include infection of the cysts, microhematuria, and calculi formation.
The average life-expectancy in patients on dialysis is lower than that of the general population with the life-expectancy ranging from 1.5 to 10 years after initiation of dialysis. Treatment for ESRD is renal transplantation and if renal cell carcinoma is detected, the treatment is surgical excision.
Pitfalls
Differentiating ACKD from other renal cyst forming diseases is based on clinical history and occurs in patients with ESRD on dialysis. Imaging features such as multiple small cysts <1 cm in size in atrophic kidneys is also helpful in making the diagnosis. Other differential considerations include cysts seen in syndromes such as tuberous sclerosis and Von Hippel Lindau disease, however, other organs are typically affected. Autosomal dominant polycystic kidney disease typically have multiple cysts of varying sizes in enlarged kidneys. Medullary sponge kidney manifests as renal cysts in the medulla of kidneys in young patients with progressive renal failure.
Teaching Points
1.
Acquired cystic kidney disease occurs in patients with end stage renal disease on dialysis and the number, size, and prevalence of cysts increase with the duration of dialysis.
2.
ACKD manifests as multiple small <1 cm bilateral cysts in atrophic to normal size kidneys in patients with end stage renal disease.
3.
These patients are at increased risk for associated complications and renal cell carcinoma and MR is an important imaging modality used to detect renal cell carcinoma.
Case 2.6
Brief Case Summary:
54 year old male with hematuria
Imaging Findings
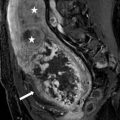
CT scan of the abdomen performed with and without the administration of intravenous contrast demonstrates the presence of a cystic mass in the interpolar region of the right kidney with the presence of an enhancing soft tissue nodule (arrows, Figs. 2.8a, b and 2.8).
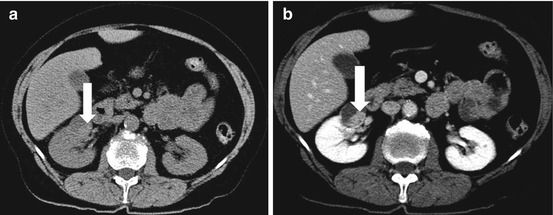

Fig. 2.8
CT scan of the abdomen performed with and without the administration of intravenous contrast demonstrates the presence of an exophytic cystic mass in the interpolar region of the right kidney with the presence of an enhancing soft tissue nodule (arrows, a, b)
Differential Diagnosis
Cystic renal cell carcinoma (Bosniak category IV).
Diagnosis and Discussion
Renal cysts are common and occur in 20–30 % of middle-aged adults and the incidence increases with age. Simple, uncomplicated renal cysts are easy to diagnose, however, renal cell carcinoma can manifest as a cystic lesion, therefore, complicated renal cysts pose a diagnostic challenge. In 1986, the Bosniak classification of renal cysts was developed to evaluate cystic renal masses, generate interobserver agreement, and decide clinical management. The Bosniak classification system is based on CT criteria but the classification system can be used with MR imaging.
Evaluation of renal masses with MR has continued to increase due to the superior contrast resolution and soft tissue characterization. The detection of enhancement is superior with MR particularly with the use of subtraction techniques to assess for enhancement. The Bosniak classification system has been applied to MR imaging with the caveat that MR can exaggerate some imaging findings. Internal septations tend to appear thicker and enhancement septations is more readily apparent thus experience in MR imaging interpretation is imperative. Additionally the quality of MR images varies by institution and MR is susceptible to more artifacts including patient motion.
The Bosniak classification system is as follows:
Category I: simple benign cysts with hairline-thin walls. The cysts do not contain septations, calcifications, or solid components. No enhancing soft tissue components are seen.
Category II: benign cystic lesions. The cysts may have hairline-thin septations with fine calcifications in the walls. Minimal enhancement of the hairline-thin smooth wall may be seen. No enhancing soft tissue components are present. Non-enhancing high-attenuation cysts less than 3 cm in size are included.
Category IIF: Complex cysts requiring follow-up imaging. These cysts are more complex and cannot fit into either category II or III. These cysts have an increased number of hairline-thin septations or mild thickening of the wall and/or septations. No enhancing soft tissue components are present.
Category III: Indeterminate masses. The cystic lesions are thickened walls and septations that are irregular. The cyst walls or septations demonstrate enhancement.
Category IV: Malignant cystic masses. Thick and irregular walls and septations may be present but enhancing soft tissue components are seen, independent of wall or septal enhancement.
Categories I and II are considered leave-alone lesions which do not require further follow-up imaging. Category IIF lesions should be follow-up imaging, however, the exact duration of follow-up imaging is uncertain. Some have proposed a minimum duration of follow-up imaging of 1–2 years. Category III lesions carry a risk of malignancy between 31 and 100 % should undergo renal biopsy and radiologic follow-up to avoid unnecessary surgery in patients with nonmalignant lesions. Category IV lesions have an incidence of malignancy between 67 and 100 % and surgical resection is the treatment of choice.
Pitfalls
Simple renal cysts are common and are benign, however, renal cell carcinoma may repsent as a cystic mass. Careful attention to the cyst walls and septations and accurate detection of enhancing soft tissue components
Teaching Point
1.
Renal cysts are common and are usually benign, but renal cell carcinoma can present as a cystic mass. Accurate characterization of cystic renal lesions is important to avoid unnecessary surgery in patients with nonmalignant lesions.
2.
The Bosniak classification system of cystic renal masses helps categorize masses based on imaging findings to assess the risk of malignancy and for evaluation of clinical treatment.
3.
Bosniak category IV cystic masses have a 67–100 % risk of malignancy and surgical resection is the preferred treatment.
Case 2.7
Brief Case Summary:
45 year old man with hematuria
Imaging Findings
Plain radiograph of the abdomen demonstrates the presence of multiple bilateral peripherally calcified masses overlying the abdomen (arrows in Fig. 2.9a). Non-contrast CT scan (Fig. 2.9b) demonstrates the presence of bilateral renal cysts, some of which have thin, mural calcifications. Both the right and left kidney are enlarged (left renal parenchyma not clearly shown on the provided CT images).
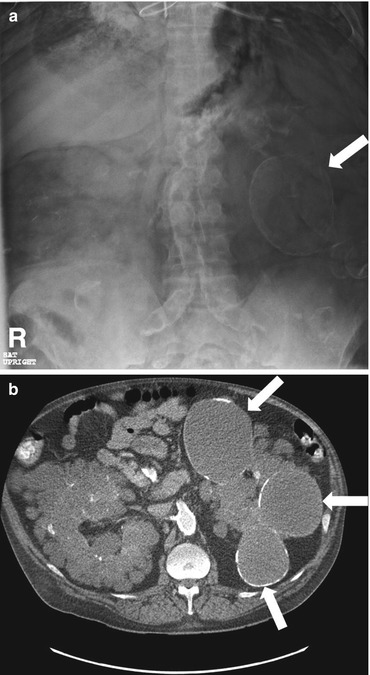

Fig. 2.9
Plain radiograph of the abdomen demonstrates the presence of multiple bilateral peripherally calcified masses overlying the abdomen (arrows in a). Non-contrast CT scan (b) demonstrates the presence of bilateral renal cysts, some of which have thin, mural calcifications. Both the right and left kidney are enlarged (left renal parenchyma not clearly shown on the provided CT images)
Differential Diagnosis
Autosomal dominant polycystic kidney disease
Multiple simple cysts
Von Hippel Lindau disease
Tuberous sclerosis
Medullary sponge kidney
Acquired cystic kidney disease
Diagnosis and Discussion:
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary cystic disease characterized by autosomal dominant inheritance but can also occur with spontaneous mutation. Patients are symptomatic and present in adulthood, between ages 30–50, with renal failure or hypertension. Renal parenchyma is normal at birth but is gradually replaced by cysts.
Patients with ADPKD can also demonstrate extrarenal cysts, most commonly in the pancreas and liver, and less commonly in the spleen, ovaries and testes. Other associated abnormalities include intracranial aneurysms, aortic and mitral valve abnormalities, colonic diverticula, aortic aneurysms and dissections. 45 % of ADPKD patients will develop end stage renal disease, the most common cause of morbidity and mortality. ADPKD patients are not at increased risk for renal cell carcinoma.
The disease is characterized by bilaterally enlarged kidneys which are replaced by multiple expansile renal cysts which may have varying attenuation, indicative of prior infection or hemorrhage. Imaging is important for diagnosis as well as the management of ADPKD with assessing complications, disease progression, and response to treatment. On CT, the kidneys are enlarged bilaterally with innumerable cysts of varying sizes. The cysts are typically large, >3 cm or larger, and are distributed throughout the renal parenchyma. On MRI, uncomplicated cysts are nonenhancing and are typically T1 hypointense and T2 hyperintense. Complicated cysts may have varying T1 and T2 signal depending on protein/hemorrhagic content. Patients with family history of cystic renal disease can be screened with ultrasound.
Patients with multiple renal cysts may have a similar appearance, however, these patients typically do not present with hypertension and/or renal failure. Family history and cysts in other organs also suggests ADPKD.
Von Hippel Lindau disease is also autosomal dominant and presents with cysts in the kidneys and may also have cysts in the other organs. However, these patients tend to have fewer cysts. These patients may also present with multiple and bilateral renal cell carcinomas which occur at a young age. Other associated lesions include retinal angiomas, cerebellar hemangiomas, adrenal pheochromocytomas and serous cystadenomas.
Tuberous sclerosis also has an autosomal dominant inheritance pattern. Patients present with multiple renal cysts in association with renal angiomyolipomas, hamartomas in the skin, brain and retina.
Medullary sponge kidney may be associated with renal failure at a young age. Cysts are typically too small to be seen. When visible, cysts occur in the renal medulla in contrast to ADPKD where cysts can appear in both medulla and cortex.
Acquired cystic kidney disease occurs in patients with chronic renal failure receiving long-term hemodialysis or peritoneal dialysis. Cysts typically occur in the cortex and rarely exceed 2 cm.
Pitfalls
Without appropriate clinical history ADPKD can be easily mistaken for other cystic renal diseases. Family history of ADPKD, hypertension and renal failure supports the diagnosis. History of chronic renal failure and long-term hemodialysis suggests uremic cystic disease.
Teaching Point
1.
ADPKD is the most common hereditary cause of renal cystic disease characterized by numerous cysts of varying sizes in bilaterally enlarged kidneys.
2.
Approximately 45 % of patients develop end stage renal disease which is the most common cause of morbidity and mortality, however, these patients are not at increased risk for renal cell carcinoma.
3.
In addition to renal cysts, ADPKD patients have extrarenal manifestations including cysts in the liver, spleen, and pancreas, intracranial aneurysms, and aortic aneurysm and dissections.
Case 2.8
Brief Case Summary:
50 year old female with systemic lupus erythematosus (SLE).
Imaging Findings
Patient is status post right nephrectomy. Axial T2 weighted MRI demonstrates a thin band of signal void along the periphery of left kidney (Fig. 2.10a). Axial non contrast CT at the corresponding slice position reveals diffuse cortical calcification extending towards the columns of Bertini with sparing of the medulla (Fig. 2.10b).
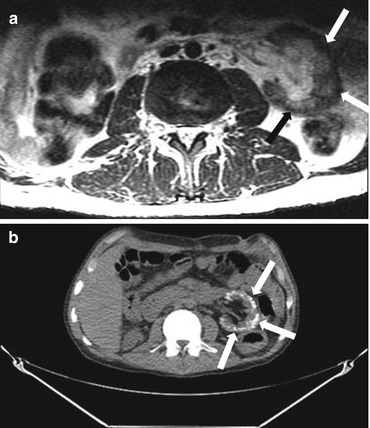

Fig. 2.10
Axial T2 weighted MRI demonstrates a thin band of signal void along the periphery of left kidney (arrow) (a). Axial non contrast CT at the corresponding slice position reveals diffuse cortical calcification (arrows) extending towards the columns of Bertini with sparing of the medulla (b)
Differential Diagnosis
Medullary nephrocalcinosis, renal tuberculosis, cortical nephrocalcinosis
Diagnosis and Discussion:
Cortical nephrocalcinosis
Nephrocalcinosis refers to deposition of calcium salts within the renal parenchyma. It may involve the cortex or medulla.
Cortical nephrocalcinosis results from diffuse cortical disease. The three most common causes include chronic glomerulonephritis, acute cortical necrosis and transplant rejection. Less common causes include chronic hypercalcemia, ethylene glycol poisoning, primary oxalosis, acquired immunodeficiency syndrome (AIDS) associated infections and sickle cell disease. In the above patient, the etiology was believed to be chronic glomerulonephritis in the setting of SLE.
On imaging, cortical nephrocalcinosis most often presents as bilateral fine renal calcifications. While these findings may be seen on plain radiographs, they are best depicted on non contrast abdominal CT. On ultrasound, it presents as increased peripheral echogenicity. On MRI, calcifications typically present as signal voids on T1 and T2 weighted images owing to susceptibility.
Three patterns of calcification have been described in cortical nephrocalcinosis:
1.
A thin peripheral band or rim of calcification beneath the capsule, which may extend into the columns of Bertini.
2.
Two parallel lines of cortical calcification known as tram track appearance.
3.
Randomly distributed punctate calcifications, likely related to calcium deposition in necrotic glomeruli.
The natural history and clinical presentation depend essentially on the underlying etiology. When detected incidentally in asymptomatic patients, further work up should be undertaken to elucidate the primary etiology, and attention directed towards treating underlying metabolic derangement.
Pitfalls
The distribution of calcification typically allows cortical nephrocalcinosis to be readily differentiated from other potential mimickers. Medullary nephrocalcinosis demonstrates calcium deposition in the medulla, while renal tuberculosis is typically unilateral and often presents with globular calcifications and/or infundibular strictures. Note that cortical nephrocalcinosis may be difficult to detect on MRI where the thin band of peripheral subcapsular signal void may be overlooked.
Teaching Points
1.
Cortical nephrocalcinosis refers to calcification within the renal cortex.
2.
Commonly causes include acute cortical necrosis, chronic glomerulonephritis and transplant rejection.
3.
Non contrast CT is the best imaging test for diagnosis. It typically reveals fine bilateral calcification, which may present as a hyperdense subcapsular band, two parallel lines of calcification within the cortex (tram track) or scattered punctuate calcifications.
Case 2.9
Brief Case Summary:
45 year old female with abdominal pain
Imaging Findings
Plain radiograph of the abdomen demonstrates the presence of bilateral calcifications overlying the region of the renal medulla (arrows, Fig. 2.11a). Gray scale ultrasound images of both kidneys demonstrate the presence of increased echogenicity in the region of the renal medulla (Fig. 2.11b, c). Coronal reformatted image of the abdomen and pelvis without intravenous contrast demonstrates the presence of bilateral renal stones distributed in the region of the renal medulla (arrows, Fig. 2.11d).
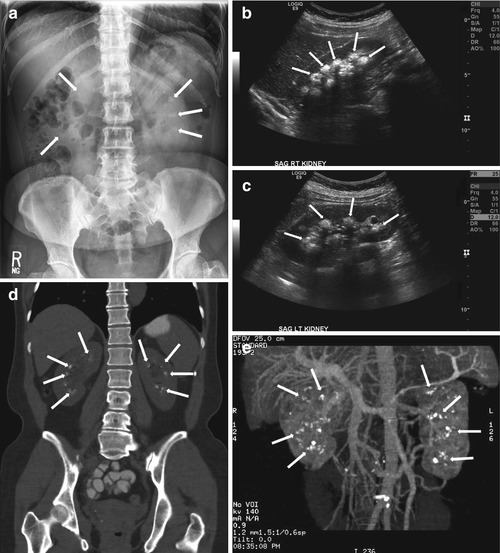

Fig. 2.11
Plain radiograph of the abdomen demonstrates the presence of bilateral calcifications overlying the region of the renal medulla (arrows, a). Gray scale ultrasound images of both kidneys demonstrate the presence of increased echogenicity in the region of the renal medulla (b, c). Coronal reformatted image of the abdomen and pelvis without intravenous contrast demonstrates the presence of bilateral renal stones distributed in the region of the renal medulla (arrows, d). Three-dimensional reformatted image in another patient demonstrates the presence of bilateral renal stones in the region of the renal medullar (e)
Three-dimensional reformatted image in another patient demonstrates the presence of bilateral renal stones in the region of the renal medulla (Fig. 2.11e)
Differential Diagnosis
Medullary nephrocalcinosis, cortical nephrocalcinosis, papillary necrosis, renal tuberculosis
Diagnosis and Discussion:
Medullary nephrocalcinosis
The most common causes of medullary nephrocalcinosis include primary hyperparathyroidism, medullary sponge kidney disease and distal renal tubular acidosis (RTA). There are two mechanisms of calcification; metastatic calcium deposition secondary to underlying metabolic derangement (as seen in hyperparathyroidism) or the precipitation of calcium salts due to urinary stasis within ecstatic distal tubules and collecting ducts (as seen in medullary sponge kidney).
Imaging findings typically demonstrate fine or coarse calcifications within the medullary pyramids. Medullary nephrocalcinosis in the setting of underling metabolic derangements is often bilateral and symmetrical while asymmetric medullary calcifications may be seen with medullary sponge kidney.
CT is very sensitive in demonstrating these calcifications which may be distributed diffusely through the pyramids (diffuse type) or along the periphery of the pyramids (rim type). On ultrasound, calcifications appear as multiple bright echogenic foci with or without distal acoustic shadowing.
Pitfalls
The distribution of calcification typically allows medullary nephrocalcinosis to be readily differentiated from other potential mimickers. Cortical nephrocalcinosis demonstrates calcium deposition in the cortex, while renal tuberculosis is typically unilateral and often presents with globular calcifications and/or infundibular strictures. Note that medullary calcifications occurring close to the tip of the pyramids may be difficult to differentiate from tiny calculi in the fornices.
Teaching Points
1.
Medullary nephrocalcinosis refers to calcification within the renal medulla.
2.
The most common causes include primary hyperparathyroidism, medullary sponge kidney disease and distal renal tubular acidosis (RTA).
3.
Imaging studies typically demonstrate calcifications within the medullary pyramids which could be diffuse or involve the periphery of the pyramids (rim type).
Case 2.10
Brief Case Summary:
48 year old female with right flank pain
Imaging Findings
Scout radiograph from a non-contrast CT scan of the abdomen and pelvis demonstrates a high density overlying the right kidney, the shape of which conforms to the right collecting system and renal pelvis (arrow, Fig. 2.12a). Non-contrast CT scan of the abdomen demonstrates the presence of a renal stone conforming to the right renal pelvis (arrow, Fig. 2.12b)
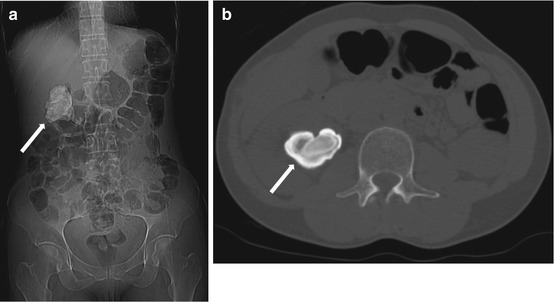

Fig. 2.12
Scout radiograph from a non-contrast CT scan of the abdomen and pelvis demonstrates a high density overlying the right kidney, the shape of which conforms to the right collecting system and renal pelvis (arrow, a). Non-contrast CT scan of the abdomen demonstrates the presence of a renal stone conforming to the right renal pelvis (arrow, b)
Differential Diagnosis
Staghorn calculus, opaque collecting system after contrast administration
Diagnosis and Discussion:
Staghorn Calculus
Staghorn calculi are branching calculi which occupy the renal pelvis and collecting system forming a cast and hence their characteristic name. They are divided into partial (involving a part of collecting system) and complete staghorn (involving almost entire collecting system). They are the result of recurrent infection and affect females more than males with predisposing factors being renal tract anomalies, VUR, neurogenic bladder or ileal ureteral diversion.
70 % of the staghorn calculi are composed of struvite (magnesium ammonium phosphate) and/or calcium carbonate apatite. Infection by urease producing bacteria (proteus, klebsiella) cause an alkaline environment in urine by breaking down urea into ammonia and hydroxide. This along with the presence of magnesium and phosphate create an environment conducive for growth of staghorn calculi. Uric acid and cystine make up for the minority of these calculi.
Imaging modalities include plain X ray KUB, ultrasound and CT.
Plain X ray KUB shows radiopaque branching laminated calcific densities in the region of renal outline. Although no consensus exists regarding definition of stag horn calculi, they are traditionally defined as partial if the renal pelvic stone extended into at least two calyceal groups or complete if at least 80 % of the collecting system is filled
The vast majority of staghorn calculi are radiopaque and appear as branching calcific densities overlying the renal outline and may mimic an excretory phase IVP. Lamination within the stone is common.
Ultrasound shows dense calcific mass filling the collecting system producing intense posterior acoustic shadowing with or without hydronephrosis.
CT shows a large branching calcific density conforming to the shape of the dilated collecting system. Their laminated appearance is best visualized on bone window which shows alternating bands of magnesium ammonium phosphate and calcium phosphate. Three dimensional images for accurate anatomical localization can be obtained which help in deciding the management. CT urography is extremely useful in delineating anatomic abnormalities of collecting system that may predispose patients to stone formation or alter therapy and for assessing complications such as pyelonephritis and urinoma due to calculus.
Recently dual source CT with advanced post acquisition processing is being used to assess the stone composition.
Although MRI holds no place in evaluation of renal calculi, it may be a useful problem solving tool in evaluating complications of calculus especially in pregnancy.
Pitfalls
An easily differentiable fallacy is presence of contrast within an opacified collecting system which may simulate the appearance of a stag horn calculus.
Teaching Point
1.
Infection by urease producing bacteria are causative organisms for formation of staghorn calculus.
2.
CT with CT urography is the modality of choice for detecting calculi and their underlying cause.
3.
Dual source CT is useful in assessing stone composition.
Case 2.11
Brief Case Summary:
A 45–year–old woman with fever and right flank pain.
Imaging Findings
Contrast enhanced CT showing focal enlargement of the right kidney with striated nephrogram. Perinephric fat stranding is noted (Fig. 2.13).
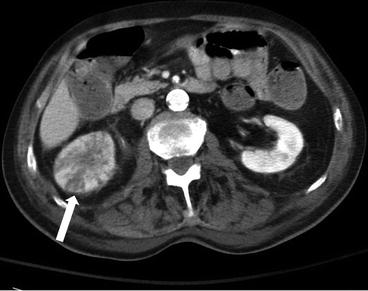

Fig. 2.13
Contrast enhanced CT showing focal enlargement of the right kidney with striated nephrogram (arrow). Perinephric fat stranding is noted
Differential Diagnosis
Pyelonephritis, infarction, nephritis caused by granulomatous diseases, acute obstruction, infiltrative neoplasm.
Diagnosis and Discussion
Acute pyelonephritis.
Acute pyelonephritis (APN) is the result of infection of renal parenchyma and pelvis which usually spreads in a retrograde fashion from lower urinary tract. Rarely it may also be caused by hematogenous spread and in these cases it is usually due to S. Aureus. Vesicoureteric reflux and underlying renal conditions like calculus disease are predisposing factors.
Not all patients suspected of APN warrant imaging. Indications of imaging include high risk patients prone for complications of acute pyelonephritis like diabetics, immunocompromised patients and in presence of obstructive calculus disease.
Ultrasound shows renal enlargement with diffuse or focal altered echogenicity – hypoechoic (edema) or hyperechogenicity (hemorrhage). Focal changes may simulate a mass. Other findings include hydronephrosis, loss of renal sinus fat and loss of corticomedullary differentiation. Local complications like presence of gas (emphysematous pyelonephritis), formation of abscess or infarct may be noted. Presence of echoes or particulate matter may be seen in the collecting system suggestive of pus or cells in urine. Doppler study shows hypoperfusion of affected segment.
CT is the modality of choice for imaging APN. The findings are best appreciated in the nephrographic phase of contrast (50–90 s after injection). If a noncontrast series is also performed it may show predisposing factors such as obstructive calculi or complications such as foci of air. Contrast CT shows wedge shaped areas in kidney, extending from renal papilla unto the cortex with focal enlargement and subtle reduction or differential contrast enhancement in corticomedullary phase as compared to the rest of the parenchyma. The periphery/cortex is also involved as opposed to renal infarct where there is sparing of the cortical rim. Focal hypodense rounded lesions may be seen. The classic sign on CT is a striated appearance with alternate areas of hypodense and normal enhancing parenchyma. Delayed phase (3–6 h post contrast) if taken shows persistent nephrogram in affected regions due to slow flow of contrast through the tubules.
CT is helpful for imaging of complications of APN as well which include renal abscess, infarction and perinephric changes of fluid, thickening of renal fascia and fat stranding.
MRI for APN is useful in patients in whom contrast material and radiation is contraindicated (e.g. contrast sensitivity and iodinated pregnancy). Imaging features are similar to CT. Gadolinium enhances conspicuity of focal lesions which remain hypointense relative to the rest of the enhancing kidney. MRI, however, lacks in detection of calcification/gas in renal parenchyma owing to unpredictable characterization.
Pitfalls
The main differential of APN is acute renal infarct and this condition can be differentiated from acute pyelonephritis by the “cortical rim sign” which signifies sparing of renal cortex vascular supply.
Teaching Point
Imaging is necessary only in high risk patients and where obstruction is suspected.
Other causes of interstitial nephritis being less common, characteristic findings in proper clinical setting point to diagnosis of bacterial pyelonephritis.
Case 2.12
Brief Case Summary:
50 year old diabetic with prior renal transplant, now complaining of acute onset right lower quadrant pain.
Imaging Findings
Ultrasound of the right lower quadrant shows the transplant kidney showing normal contour, the pelvicaliceal system displays echogenic contents with reverberation and ring down artefacts (“dirty shadowing”) suggesting gas pockets (arrows in Fig. 2.14a).
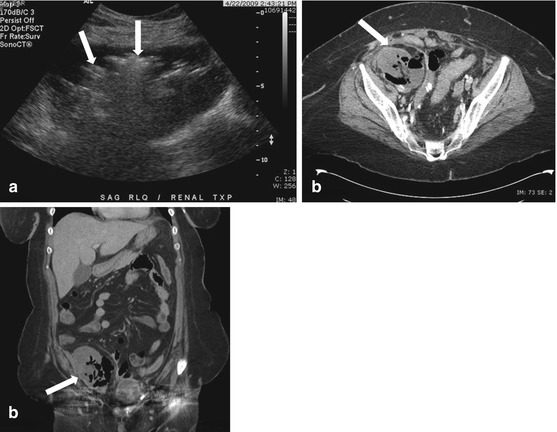





Fig. 2.14
Ultrasound of the right lower quadrant shows the transplant kidney showing normal contour, the pelvicaliceal system displays echogenic contents with reverberation and ring down artefacts (“dirty shadowing”) suggesting gas pockets (arrows in a). Axial and coronal CT scan images (b, c) confirms these findings of locules of gas within the collecting system of the transplant kidney (arrow), in addition, it demonstrates gas within renal parenchyma as well. A perinephric collection is also shown to contain gas pockets
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree