LARYNX, HYPOPHARYNX, AND CERVICAL ESOPHAGUS
KEY POINTS
- CT and MRI are important tools for establishing the entire extent of a primary laryngeal cancer much of which that is often beyond endoscopic detection in locally advanced cancers
- CT and MRI are important tools for establishing the extent of cervical and retropharyngeal lymph node metastases
- These two factors help determine optimal treatment strategies
- FDG PET used judiciously can further aid this diagnostic process
- Surveillance imaging can be by a combination of anatomic and/or radionuclide imaging- the best strategies are individualized to each particular patient’s circumstances
- CT and MRI are important tools for differentiating radionecrosis of the laryngeal skeleton from recurrence and determining the possible threat of radionecrosis to airway integrity
INTRODUCTION
Staging and medical decision-making procedures for laryngeal cancer include indirect laryngoscopy, direct laryngoscopy with biopsy, contrast-enhanced computed tomography (CECT) or contrast-enhanced magnetic resonance (CEMR) depending on the practice preference, and a chest x-ray. Imaging studies are an essential component of the clinical diagnostic evaluation, providing unique and often pivotal information that complements the clinical examination. Imaging provides additional information concerning the deep extent and regional spread of disease. Chest computed tomography (CT) and fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) may provide additional information in selected cases.
This chapter describes the important role of diagnostic imaging in clinical decision making for laryngeal cancer. Squamous cell carcinoma (SCCA), the most common malignancy, is considered in the first section, and the less common malignant tumors are presented in the second section.
ANATOMIC AND DEVELOPMENTAL CONSIDERATIONS
Applied Anatomy
A thorough knowledge of laryngeal anatomy and anatomic variations in each of the following areas is required for the evaluation of laryngeal malignant tumors. This anatomy is presented in detail in the following introductory chapters:
Primary Site Evaluation
 Larynx, including the laryngeal skeleton, deep tissues spaces within the larynx, mucosal landmarks, and functional structures within the larynx (Chapter 201)
Larynx, including the laryngeal skeleton, deep tissues spaces within the larynx, mucosal landmarks, and functional structures within the larynx (Chapter 201)
 Hypopharynx (Chapter 215)
Hypopharynx (Chapter 215)
 Tongue base region and low oropharyngeal wall (Chapter 190)
Tongue base region and low oropharyngeal wall (Chapter 190)
 Cervical esophagus, including the critical esophageal verge at the junction of the postcricoid portion of the hypopharynx and cervical esophagus (Chapter 221)
Cervical esophagus, including the critical esophageal verge at the junction of the postcricoid portion of the hypopharynx and cervical esophagus (Chapter 221)
 Trachea (Chapter 209)
Trachea (Chapter 209)
Evaluation of Extralaryngeal Spread
 Visceral compartment of the neck and related fasciae (Chapter 149)
Visceral compartment of the neck and related fasciae (Chapter 149)
Evaluation of Regional Lymph Nodes Disease
 Detailed knowledge of normal node and perinodal morphology and the common drainage pathways of laryngeal cancer, which emphasizes nodal levels 2 through 6 (Chapters 149 and 157)
Detailed knowledge of normal node and perinodal morphology and the common drainage pathways of laryngeal cancer, which emphasizes nodal levels 2 through 6 (Chapters 149 and 157)
Evaluation of Perivascular and Perineural Spread
 Knowledge of the entire course of the vagus and recurrent laryngeal nerves on both sides and of the superior and inferior laryngeal neurovascular bundle (Chapter 201)
Knowledge of the entire course of the vagus and recurrent laryngeal nerves on both sides and of the superior and inferior laryngeal neurovascular bundle (Chapter 201)
IMAGING APPROACH
Techniques and Relevant Aspects
Specific CT and magnetic resonance (MR) techniques for study of the larynx are reviewed in Chapter 201, and specific protocols for laryngeal malignant tumors and related problems are presented in Appendixes A and B. A few points of emphasis related to malignant laryngeal tumors follow. The neck should always be slightly hyperextended for laryngeal CT to reduce shoulder-related artifacts. Laryngeal images must be viewed as parallel to the true vocal cords (TVCs). Sections made or viewed oblique relative to the TVC can produce substandard images that lead to interpretative difficulties. In particular, review of skewed images may lead to an errant interpretation of the anatomic relationships in the transglottic region that are often critical to medical decision making.
Intravenous contrast must be used for all studies of laryngeal cancer unless there is a compelling contraindication. Many primary tumors will enhance, improving the tumor-to-muscle contrast, and intravenous contrast use is critical in the evaluation of altered nodal morphology and likely perfusion as an indication of metastasis. If iodinated contrast cannot be used, then magnetic resonance imaging (MRI) should be considered as the primary study for any lesion except a limited glottic cancer. The use of intravenous paramagnetic contrast is individualized, but generally it is necessary.
Pros and Cons
Indications for Study
Evaluation of the Primary Site and Regional Disease
T1 lesions of the TVC do not usually require imaging. Any tumor that is suspicious for deep infiltration should be studied primarily with CT (Fig. 206.1). Supplemental MR study is directed by CT findings to a localized area of interest where its strengths related to better soft tissue contrast resolution may be exploited (Fig. 206.2). A large proportion of MR studies will be of poor quality owing to motion artifacts.
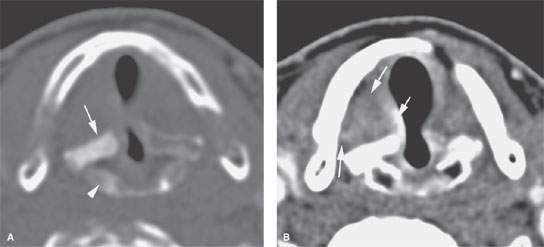
FIGURE 206.1. A patient with irregularity of the right true vocal cord noted clinically. Computed tomography study in (A) shows a sclerotic arytenoid (arrow) and top of the cricoid cartilage (arrowhead). These findings are suspicious for at least chronic inflammation and likely adjacent tumor. Multiple biopsies showed only dysplasia. The patient was re-biopsied, and carcinoma was diagnosed. In (B), the vocal cords are apposed because the patient was holding his breath. There was slight bulge of the posterior cord and some thickening of the true cord, but the paraglottic fat is preserved. Findings were consistent with a very limited superficial lesion. The patient responded completely to radiotherapy.
All primary tumors must be evaluated for the extent of the endolaryngeal spread of the primary; cartilage invasion; spread to adjacent structures including the hypopharynx, tongue base, trachea, and esophagus; and extralaryngeal spread to the soft tissues of the neck. The evaluation must also include a search for regional adenopathy and spread along neurovascular bundles at risk. Finally, the risk of any acute adverse complication should be anticipated, such as the lesion really representing one of vascular origin that would not be optimal for a deep biting type of tissue sampling.
There are several areas where CT or MR images consistently provide better information than the endoscopic examination. These include spread to the subglottis, paraglottic space, pre-epiglottic space, tongue base via the pre-epiglottic space, and cartilage invasion. These imaging studies also allow tumor volumes that may reside in these deep spaces to be measured along with the more exophytic component.
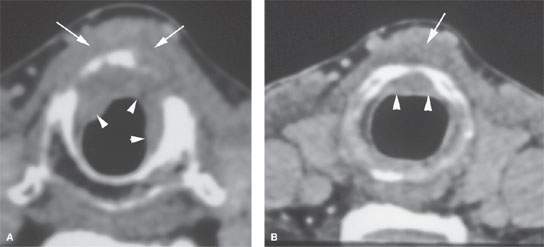
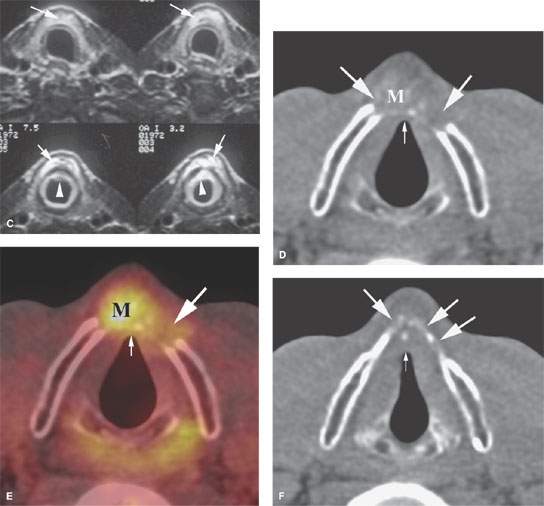
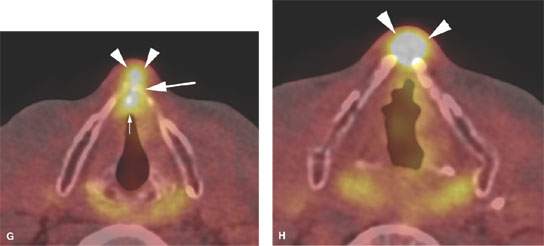
FIGURE 206.2. A–C: Patient 1 with subglottic carcinoma. The computed tomography (CT) images in (A) and (B) show the endoluminal portion of the tumor (arrowheads) but also show spread of tumor anterior to the cricoid cartilage (arrows). In (C), the magnetic resonance images again clearly show the endoluminal component (arrowheads) but also more definitely demonstrate the tumor spreading beneath the infrahyoid strap muscles (arrows) so low that the tracheostomy in this patient had to be placed lower than usual. D–H: Patient 2 with an fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography (PET–FDG) study that graphically depicts cartilage invasion and extralaryngeal spread of tumor but contributes no additional information that substantively changes the approach to treatment that would be based on the anatomic images. A non–contrast-enhanced axial CT image at the level of superior cricoid cartilage (D) demonstrates mild thickening of the anterior commissure (small white arrow) and gross destruction of the thyroid cartilage bilaterally (large arrows) that is caused by a heterogeneous-appearing mass (M). The mass grossly infiltrates the prelaryngeal soft tissues. The fused axial PET CT image at the same level (E) shows markedly increased FDG uptake in the heterogeneous-appearing mass (M) and slightly increased FDG uptake at the anterior commissure (small white arrow) and site of thyroid cartilage destruction (large arrow). The non–contrast-enhanced axial CT image at the level of the arytenoids cartilages (F) shows persistent marked thickening of the anterior commissure (small white arrow). The anterior portions of the thyroid cartilage bilaterally (large arrows) are present but markedly less mineralized in nature than the remaining portions of the thyroid cartilage. Since no mass is seen at this level, it is unclear if the patchy mineralization is caused by tumor infiltration or represents physiologic variation in thyroid cartilage mineralization.
Detection of Recurrent Tumor
Diagnostic CT is the examination for detection of recurrence in the neck, study of the neopharynx, or the irradiated larynx; in selected cases, positron emission tomography (PET) may be useful, as discussed in the subsequent section on follow-up surveillance.
Evaluation of Laryngeal Dysfunction and Submucosal Masses
Some patients have SCCAs growing entirely beneath intact mucosa. These malignancies may begin on the mucosa within the laryngeal ventricle and the false vocal cord (FVC) and grow in the paraglottic space (Fig. 6.3). Submucosal masses caused by an enchondroma or chondrosarcoma are more specifically diagnosed by CT than MRI (Figs. 205.6 and 205.7 and Chapter 39). Other submucosal cancers from minor salivary and benign mesenchymal tumors cannot be distinguished from SCCAs (Chapter 205). A rare paraganglioma might be anticipated by intense vascularity and an enlarged superior laryngeal vascular bundle (Fig. 205.2). About half of all submucosal laryngeal masses will be laryngoceles. The presence of a laryngocele requires a search for an obstructing primary tumor (Chapter 203). Deformity of the laryngeal skeleton due to old trauma or developmental variations may cause laryngeal dysfunction and a mucosal bulge. This condition is readily recognized (Figs. 201.35, 201.36, and 205.7E and Chapter 201). All of these conditions that may produce a submucosal laryngeal mass are discussed in more detail elsewhere.
Dysphonia, Vocal Cord Dysfunction, and Palsy
TVC dysfunction that is believed clinically to be due to recurrent laryngeal or vagal neuropathy will on occasion be caused by an entirely submucosal laryngeal pathology; the most frequent cause is cancer (Fig. 206.3).
CT is the preferred imaging modality since it not only provides a good evaluation of the larynx but also can easily be extended to include the upper thoracic cavity or intracranial structures when the cause is not endolaryngeal, as discussed in Chapter 201. Adjunctive MRI may be used selectively to evaluate the cisternal course of the vagus nerve and its brain stem nuclei.
Controversies
Cartilage Invasion
CT and MR vastly improve the clinical detection of subtle cartilage invasion. A slice thickness, 3 mm (preferably 0.5 to 1.0 mm) is required for this task, so CT is the better choice as the primary examination. There have been suggestions that MR is better than CT for detection of early cartilage invasion. CT and MR are both imperfect in detecting early macroinvasion and, of course by definition, cannot directly show microinvasion. Variation in ossification of the cartilages and the related marrow spaces makes interpretation difficult. Volume averaging aggravates the effects of these anatomic variations. Foci of cartilage/bone invasion .3 to 5 mm should be visible or strongly suggestive of early macroinvasion if imaging techniques are adequate. CT sections, 3 mm thick and reformations facilitate the search for small defects and early marrow space invasion (Fig. 206.4).
The contrast resolution of MR can show small areas of marrow space invasion. Unfortunately, replacement of fat in the marrow space of the thyroid cartilage can be due to reactive fibrovascular proliferation, caused by a tumor adjacent to but not invading the cartilage in question1 (Fig. 206.5). This leads to false-positive interpretation of both CT and MR studies as suggesting minimal macro- or microinvasion. Such false-positive readings may lead to unwarranted upward stage migration, since tumors with thyroid cartilage invasion are designated as T4 tumors. More importantly, it could cause needless loss of the larynx in the hands of those not thoroughly familiar with these pitfalls and limitations of imaging. On the other hand, asymmetries in the cartilage should alert the radiologist to scrutinize the adjacent laryngeal soft tissues for signs of tumor infiltration (Figs. 206.1 and 206.4).
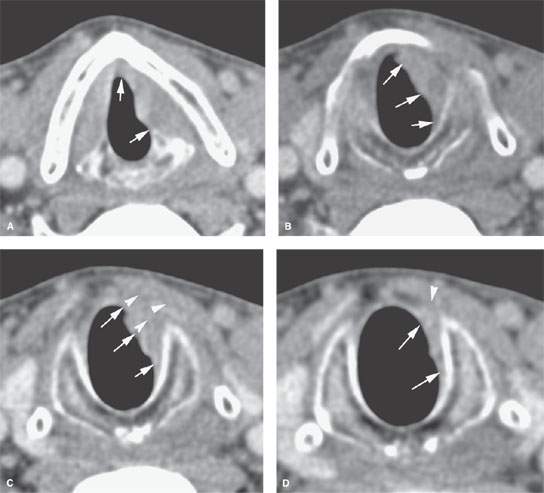
FIGURE 206.3. A patient presenting with what was believed to be true vocal cord paralysis. Computed tomography study from the posterior fossa to the aortopulmonary window showed no abnormality along the vagus or recurrent laryngeal nerves. It did, however, show an infiltrating tumor of the larynx that was entirely submucosal. A: The tumor involves the entire true cord up to the anterior commissure (arrows). B: Subglottic spread is present (arrows). C: The subglottic spread (arrows) extends through the cricothyroid membrane into the soft tissues beneath the infrahyoid strap muscles (arrowheads). D: Continued subglottic spread is present almost to the bottom of the cricoid cartilage (arrows).
Early cartilage invasion is best assessed by a combined commonsense and objective consideration of factors that can assign risk of invasion and relate it to treatment outcome. In taking such a risk-profiling approach, cartilage invasion may be only one of several factors influencing outcome. Some basic factors about the primary tumor can help to assess early cartilage invasion. These include site of origin and volume of the primary tumor; known proclivity of primary tumors at that site relative to cartilage invasion; infiltrating versus pushing tumor margins; degree of cartilage ossification adjacent to tumor; whether cartilage ossification is symmetric; sclerosis of cartilage adjacent to tumor; focal or diffuse obliteration of the fatty marrow space; possible invasion of connecting membranes connecting cartilages; and edema of extralaryngeal soft tissues manifesting, in early cases, as loss of the fat plane between the infrahyoid strap muscles and the cartilage if such a plane is present in the patient.
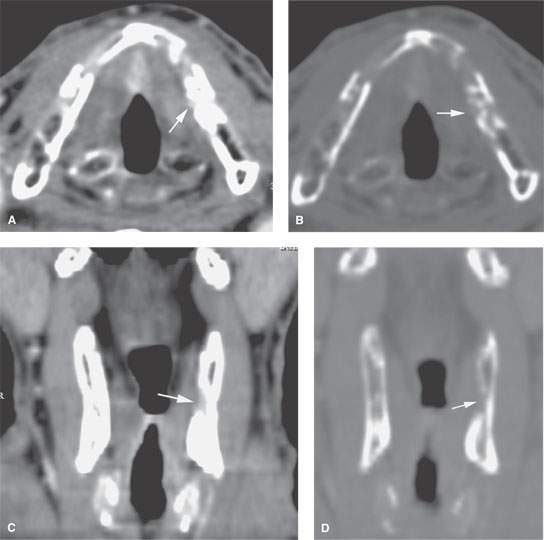
FIGURE 206.4. A patient with an early transglottic tumor and computed tomography (CT) demonstrating probable focal thyroid cartilage invasion. The case also illustrates the excellent quality reformations that are currently available with multidetector CT. A: Tumor of the true cord thickens the anterior commissure and obliterates the paraglottic fat at a level just above the laryngeal ventricle (arrow). B: Bone window settings show the focal defect in the thyroid cartilage more clearly suggesting very early cartilage invasion (arrow). C, D: Coronal reformations confirm the presence of a defect in the low paraglottic space along the inner margin of the thyroid lamina (arrows). Such defects can be normal variants; they might also represent early cartilage invasion. (NOTE: The significance of very minimal cartilage invasion with regard to an impact on survival is uncertain and at this time doubtful. Refer also to Fig. 206.5 for correlating information.)
Cartilage invasion has, for years, been a putative marker for a tumor at an increased risk of failing radiotherapy (RT). This belief was justifiable in the pre-CT era when detection of cartilage invasion depended on palpation and plain film evidence of cartilage erosion. By the time that cartilage invasion was detected using these now primitive diagnostic tools, however, the patient was clearly beyond cure with RT. The detection of early cartilage invasion by CT and MR provides more accurate tumor staging information and can be used to determine the best treatment modality. Cartilage invasion or an increased risk of cartilage invasion should be part of a risk profile in selecting the strategy for cure, preserving as much laryngeal function as possible.2
Subtle macroinvasion may be evaluated as described previously in this discussion; however, there will always be false-positive and -negative studies in this regard. Risk of micro- or macroinvasion can be assessed by simple imaging parameters. The concept was first introduced by Archer et al.3 using tumor axial dimensions and positions. Such a risk profile for micro- or early macroinvasion can be even more productively linked to a predictive model for treatment outcome. This method relies only on generating a tumor volume and observing whether the cricoid, arytenoids, and/or thyroid cartilages adjacent to tumor are sclerotic. This method can stratify patients with T3 glottic cancers into high, medium, and low risk of failure with RT alone.4 Although the risk of failure may be related to laryngeal framework invasion, its prognostic impact has not been proven (Figs. 21.44, 206.4, and 206.5).
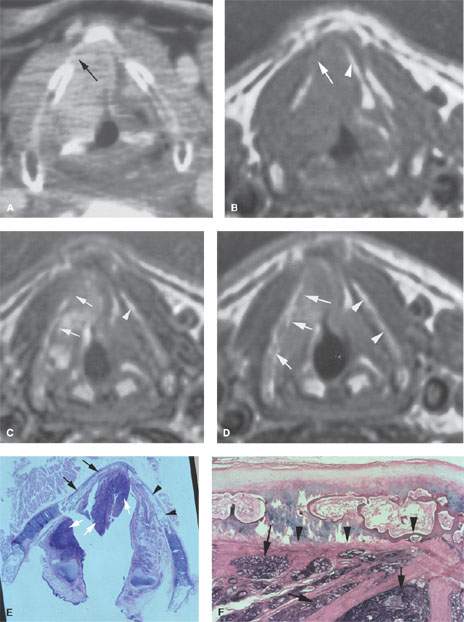
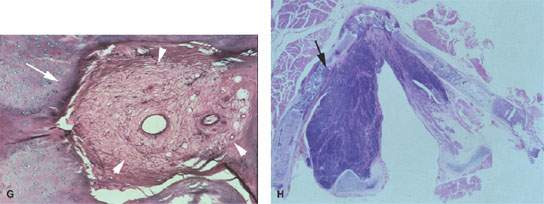
FIGURE 206.5. Patient with false vocal cord carcinoma. A: Computed tomography showing possible thyroid cartilage invasion (arrow). B: The non–contrast-enhanced T1-weighted (T1W) image again is suspicious for invasion with possible obliteration of fat within the thyroid cartilage (arrow) compared to the fat within the thyroid lamina on the normal side (arrowhead). C: Contrast-enhanced T1W image showing irregularity and enhancement along the thyroid lamina suspicious for invasion compared to the appearance of the lamina on the normal side (arrowheads). D: A T2-weighted image again suggests infiltration of the marrow space of the thyroid lamina (arrows) compared to the normal side (arrowhead). E: Whole organ section on this patient through the false cord level. Tumor (white arrows) is present in the soft tissues; however, the thyroid cartilage is not invaded. The thyroid lamina on the right (arrows) is not as well ossified as on the left (arrowheads), where it is obviously ossified and contains a fatty marrow space. F: Close-up photomicrograph showing no evidence of tumor invading the perichondrium interface with the thyroid lamina either at cartilaginous portions of the thyroid lamina or at ossified portions (arrowheads). Tumor obviously infiltrates the deep soft tissues (arrows). G: Multiple areas of fibrosis (arrowheads) were found within the cartilage of the right thyroid lamina. Such reactive changes are sometimes incited by adjacent tumor and mimic cartilage invasion. This patient could likely have been controlled by radiotherapy rather than laryngectomy based on these findings. H: Photomicrograph of a whole organ section of Patient 3 showing a small area of cartilage invasion (arrows) similar to that seen in Figure 206.4. It is uncertain whether recognizing such minimal areas of cartilage invasion are a benefit or a detriment to strategies that aim to conserve laryngeal function while improving survival in patients with laryngeal carcinoma. (Material in this figure courtesy of Minerva Becker, M.D., Geneva, Switzerland.)
CT and MRI easily identify tumor growing outside of the larynx, usually via the thyrohyoid and cricothyroid membranes/ligaments to the soft tissues of the neck. Such spread is normally easy to distinguish from cervical lymph nodes metastases. Spread to the thyroid gland, pharynx, and cervical esophageal verge is also assessed.
FDG-PET for Staging
In most laryngeal neoplasms, FDG-PET has a modest, if any, primary role to play (Fig. 206.2D–H), as this technique does not allow one to reliably assess the anatomic extent of the lesion and the possible invasion of the tumor into adjacent structures. Its value in determining or planning gross tumor volume for RT planning is controversial, especially in smaller laryngeal cancers.
FDG-PET can detect neck nodal metastasis with relatively high accuracy, but both false-positive and false-negative results occur. Although FDG-PET sometimes detects nodal metastasis missed by other imaging techniques, the high cost of this technique does not warrant its routine use for this indication. It is certainly not an appropriate routine study in patients with T1 and T2 glottic cancers.
It may be warranted in other circumstances in the patient population with these cancers to detect distant metastases and coincident primary tumors outside the head and neck region. This technique must be used selectively rather than applied indiscriminately for diagnosis and staging given its expense and often low potential to truly alter management or outcome.
In advanced laryngeal cancer (T4) or in patients with adenopathy low in the neck (levels 4, 5B, or 6), adding an FDG-PET study may be useful to exclude distant metastasis. In tumors requiring very extensive resections, FDG-PET is also useful to exclude a second primary tumor to avoid unnecessary mutilation of the patient.
In the posttreatment situation, FDG-PET may sometimes be helpful as discussed in a following section surveillance options.
Tumor response to chemotherapy as measured by FDG-PET is being investigated as a way to select patients who would benefit from chemoradiation organ preservation therapy versus surgery and postoperative RT treatment strategies. This application is not yet proven to be beneficial, as discussed in Chapter 21 (Fig. 21.63). Tumor perfusion may eventually be a better parameter in this regard.
SPECIFIC DISEASE/CONDITION
Laryngeal Squamous Cell and Other Carcinomas
Etiology
SCCA of the larynx is clearly linked to cigarette smoking. Alcohol is a contributing and likely potentiating risk factor but probably less so than in cancer in other head and neck sites. No other etiologies have been proven. Other carcinomas are of predominantly glandular origin and are sporadic and not necessarily linked to known predisposing factors.
Prevalence and Epidemiology
Laryngeal carcinoma is a common disease in the smoking population. There is about 10:1 male to female predominance.5 Localized and regional disease accounts for the vast majority of cases at presentation, with distant metastasis being uncommon of patients at the time of initial diagnosis. Glottic cancer is more prevalent than supraglottic cancer. The rates of occurrence are increasing in both males and females but proportionally much more in females.
Clinical Presentation
TVC cancer produces hoarseness as the initial symptom. Sore throat, ear pain, pain localized to the thyroid cartilage, and airway obstruction may occur in advanced lesions. Glottic and subglottic cancers occasionally present with stridor or airway symptoms.
Hoarseness is usually not a presenting symptom in patients with supraglottic cancer but may occur in the presence of advanced disease. Changes in voice quality do occur. Dysphagia and referred otalgia (from the vagus to the Arnold nerve) are common presenting complaints. In supraglottic cancer, a neck mass due to metastatic adenopathy is a common presenting sign.
Physical examination is mainly done by rigid and flexible fiber-optic endoscopes. The physical examination should establish whether the vocal cord is mobile, paretic, or fixed.
Ulceration of the infrahyoid epiglottis and/fullness of the vallecula may indicate pre-epiglottic space invasion. Fullness to palpation of the thyrohyoid membrane region may also suggest invasion of the pre-epiglottic space.
The laryngeal click, which can be elicited by media to lateral manual manipulation of the larynx over the vertebral column, may become absent in the presence of postcricoid spread.
Localized pain or tenderness to palpation over the lamina suggests gross invasion of the cartilage.
Pathology
The laryngeal surfaces of the epiglottis and TVCs are lined with stratified squamous epithelium. Otherwise, the larynx is lined with pseudostratified ciliated columnar epithelium. This squamous cell surface epithelium is the source of nearly all squamous laryngeal malignancies. Benign and malignant minor salivary gland tumors arise from the mucous glands deep to the laryngeal mucosa.
Most of the vocal cord carcinomas are either well differentiated or moderately well differentiated. Sometimes there is an apparent carcinoma and sarcomas occurring together, but these tumors are usually squamous carcinomas with a pseudosarcomatous or spindle cell stromal reaction. Spindle cell carcinomas in the larynx and trachea may present as polypoid or pedunculated masses that have a very favorable prognosis. Verrucous carcinoma occurs in a very small percentage of patients, but histologic diagnosis is difficult and must correlate with the gross appearance of the lesion. Supraglottic carcinomas tend to be less differentiated than TVC primaries. Small cell carcinoma (Fig. 30.14) is rarely diagnosed in the supraglottic larynx. It is biologically very aggressive with a high risk for distant spread.
General Common Pathways and Trends
Generally, tumor growth in the larynx is directed by existing natural anatomic barriers. A tumor margin may be infiltrative or have pushing, well-defined borders as discussed in Chapter 21. There is a tendency for supraglottic and glottic lesions to remain, at least for a time, confined to their compartments of origin. However, there is no real anatomic barrier that restricts spread from one area to the next.
Glottic cancers tend to be slow growing but may extend to the supraglottis and subglottis over time. Because supraglottic cancers often arise well above the TVC, TVC involvement is usually a late phenomenon. Supraglottic cancer may spread to the TVC level via the paraglottic space deep to the FVC and laryngeal ventricle mucosa. The fibrous ligament at the anterior commissure is a relative barrier to midline submucosal tumor spread from the epiglottis to and below the anterior commissure; thus, involvement is mainly a surface phenomenon as seen in the anterior, inferior spread of supraglottic cancers.
The pre-epiglottic and paraglottic spaces are predominantly fatty. The quadrangular membrane may have a minor role in directing submucosal tumor spread for infrahyoid epiglottic, FVC, and TVC lesions (Fig. 206.6A–C). As the FVC and TVC tumors spread laterally, they reach the thyroid cartilage and are most often deflected growing superiorly and inferiorly within the paraglottic space (Fig. 206.7). Sometimes the cancer grows through the cricothyroid and thyrohyoid membranes before invading the thyroid lamina (Fig. 206.8). Tumor headed inferiorly within the paraglottic space is commonly diverted by the conus elasticus (Figs. 201.3–201.8). Tumor often grows toward and then through the cricothyroid membrane and into the tissues of the neck, where it may invade the thyroid gland. Such spread beyond the laryngeal skeleton is most often contained by the infrahyoid strap muscles. In advanced cases, tumor may grow across the visceral compartment fascia to invade the superficial fascia and eventually the subcutaneous fat and skin.
Thyroid cartilage invasion usually begins in an ossified section of the cartilage (Figs. 206.4 and 206.5). The specific access for invasion of the ossified cartilage is often along areas where there are small fissures, created by penetrating ligaments and connecting membranes and neurovascular bundles. This likely explains why glottic cancer involving the anterior commissure invades the thyroid cartilage at the point of attachment of the vocal ligament (Fig. 206.9). Cancer spread directed inferiorly by the conus elasticus and cricothyroid membrane will invade their attachments along the upper margin of the cricoid and lower margin of the thyroid cartilage. Supraglottic cancers only infrequently invade the adjacent thyroid lamina. Such invasion is usually at the insertion of the thyrohyoid membrane along the upper margin of the thyroid lamina. Tumor spreading in the paraglottic space often has a broad zone of contact with the perichondrium of the thyroid cartilage (Fig. 206.10). However, direct invasion of the adjacent lamina away from its edges and sites of membrane and muscle insertions is uncommon and almost assuredly is due to invasion along vessels penetrating the ossified portion of the thyroid lamina.
Micheau et al.6 studied 120 cases of T2 through T4 laryngeal cancers without preoperative radiation. In the 70 patients with cartilage invasion, 66 patients had invasion that occurred at the ossified sites. The authors noted that the most common site of thyroid cartilage invasion occurs at the anterior angle and that apparently discontinuous spread within the marrow space of an ossified thyroid lamina occurs.
Archer et al.,3 in the early days of laryngeal CT, showed that when the average diameter of the tumor mass was .16 mm and the lesion center was located below the superior process of the arytenoid cartilage, 12 of 14 patients had cartilage invasion on pathologic examination. Nine of the 12 had three cartilages invaded. Cartilage invasion could only be detected by microscopic examination in 8 patients. This experience presaged the concept developed about 5 to 10 years later that tumor volume combined with arytenoid, thyroid, and/or cricoid cartilage sclerosis is a reasonable way to predict microscopic cartilage invasion, with the surrogate outcome for likely cartilage invasion in these later studies being risk of RT failure in T3 glottic cancers. This was first suggested by Lee et al.7 and then refined by Pameijer et al.4 Imaging-based risk stratification has shown that fixed-cord T3 glottic cancer with volume, 3.5 cc and no cartilage sclerosis could be controlled by RT alone in .90% of cases. Those with tumor volume of $3.5 cc and sclerotic changes of one of the three cartilages had a 40% to 60% chance of control with RT alone. If tumor volume was .3.5 cc and two or three cartilages were sclerotic, there was a 90% risk of failure with RT alone. A separate investigation8 of cricoid cartilage abnormalities in glottic carcinoma yielded a statistically significant lower control rate after RT. Ten out of the 13 patients with sclerosis of the cricoid in this study also had sclerosis of the arytenoid cartilage, corresponding with the “double sclerosis” situation (arytenoid and cricoid cartilage involvement) described by Pameijer et al.4 However, when multivariate analysis was performed, cartilage abnormalities were no longer predictive of failure when paraglottic and pre-epiglottic space involvement were included in the analysis. Even relatively subtle cartilage abnormalities are often correlated with deep tumor extension. Deep involvement of the paraglottic space at the glottic level, as seen on imaging studies, is also referred to as the “adjacent sign” due to tumor growing adjacent to the thyroid cartilage. This sign was found to be the only independent predictor of local outcome and survival in a series of 130 patients with T1 and T2 glottic cancer.9 It appears that cartilage abnormalities are an epiphenomenon of large tumor volume and deep tumor extension, which are probably better predictors of local outcome after RT.
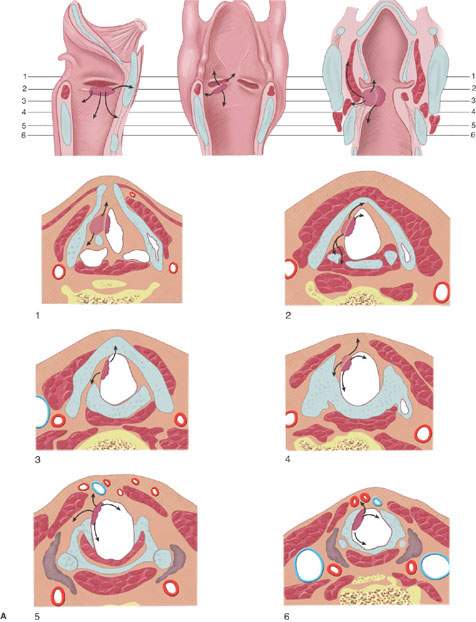
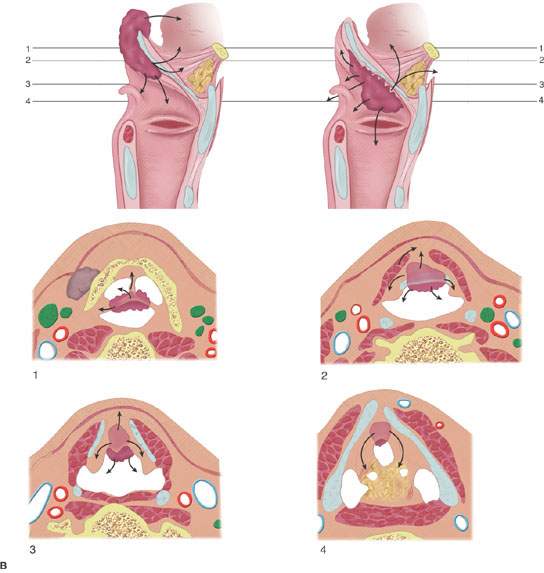
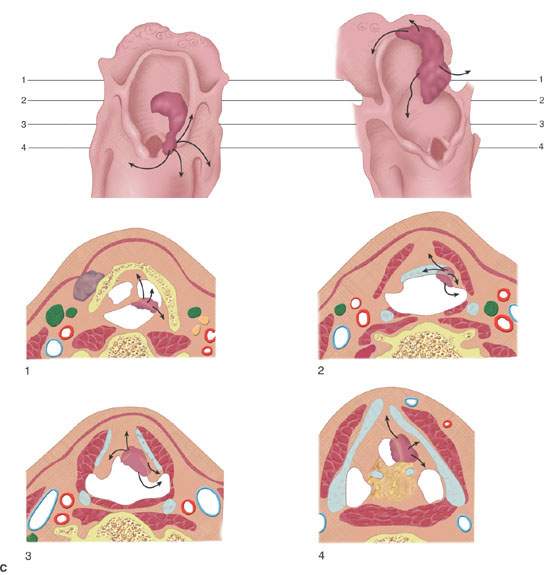
FIGURE 206.6. Spread patterns of laryngeal cancer. A: Glottic carcinoma spread patterns. B: Spread patterns of epiglottic cancer both of the infrahyoid and suprahyoid epiglottis. C: Spread patterns of marginal supraglottic lesions including those of the aryepiglottic fold, false cord, and laryngeal ventricle mucosa.
Other evidence of deep tumor invasion is fixation of the vocal cord. This is usually caused by invasion or destruction of the vocal muscles, invasion of the cricoarytenoid joint, or rarely invasion of the recurrent laryngeal nerve. Perineural spread is uncommon in laryngeal malignancies.
Supraglottis
Supraglottic cancers are often epiglottic in origin. However, it might be difficult to precisely tell the subsite of supraglottic origin of advanced lesions except by its appearance on CT or MR images. Even with those tools, it is sometimes difficult.
Suprahyoid Epiglottis
Lesions of the suprahyoid epiglottis (Fig. 206.6B,C) may appear predominantly as an exophytic mass with little inclination to destroy the epiglottic cartilage or spread to adjacent structures or deep spaces. Some may infiltrate the free margin of the epiglottis, destroying the cartilage and amputating its tip. These latter cancers tend to invade the mucosa of the vallecula and lateral pharyngeal walls (Fig. 206.11). To some extent, the predominantly superficial or deep spread of epiglottic cancer may be directed by the quadrangular membrane, which is a relatively weak barrier to tumor spread.
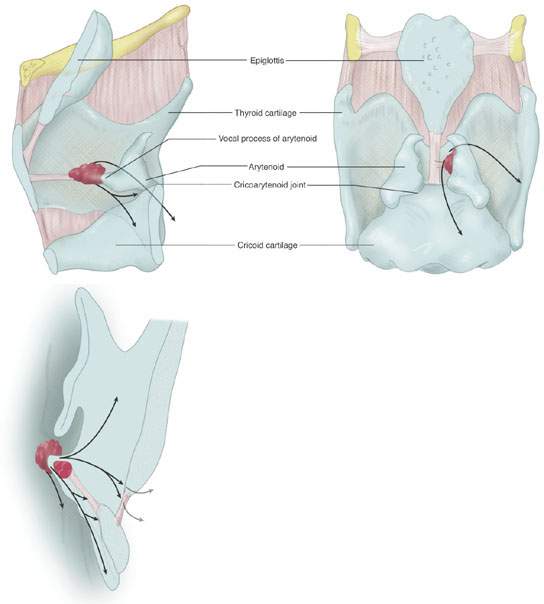
FIGURE 206.7. Spread patterns of glottic carcinoma, especially with regard to threats to the cricoid and arytenoid cartilages and occult patterns of deep spread within the paraglottic space.
Infrahyoid Epiglottis
Lesions of the infrahyoid epiglottis (Fig. 206.6B,C) produce irregular nodular mucosal masses often showing simultaneous invasion through the porous epiglottic cartilage and thyroepiglottic ligament into the pre-epiglottic space (Fig. 206.12). Tumor may spread superiorly within the pre-epiglottic space and involve the vallecula and base of the tongue without involving the suprahyoid epiglottis or showing any other clinically obvious mucosal contiguity (Fig. 206.13).
Cancers of the infrahyoid epiglottis also grow circumferentially to involve the aryepiglottic folds and thereby the medial wall of the pyriform sinus. Inferolateral growth will eventually reach the false vocal folds, and superolateral growth will extend to the pharyngoepiglottic fold. Invasion of the anterior commissure and TVCs is usually a late occurrence, and subglottic extension occurs occasionally in similarly advanced lesions. Such “transglottic” growth is much more typical of FVC cord and ventricular primaries. Infrahyoid epiglottic lesions that grow in a transglottic manner are at high risk for thyroid cartilage invasion, even if the vocal cords are mobile.
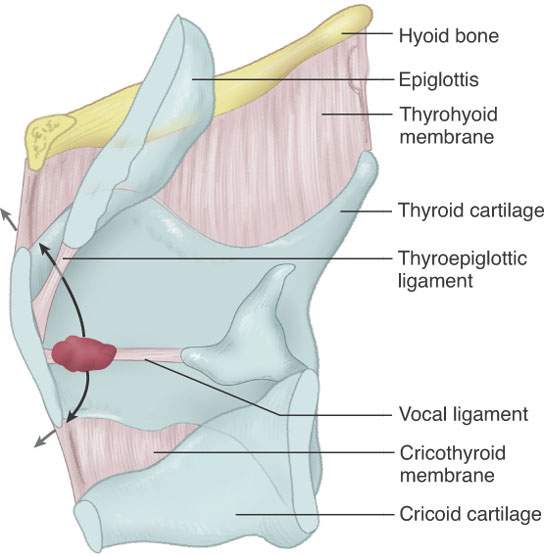
FIGURE 206.8. Patterns of spread of cancers arising at or near the anterior commissure and in particular their tendency to spread early to involve the laryngeal skeleton.
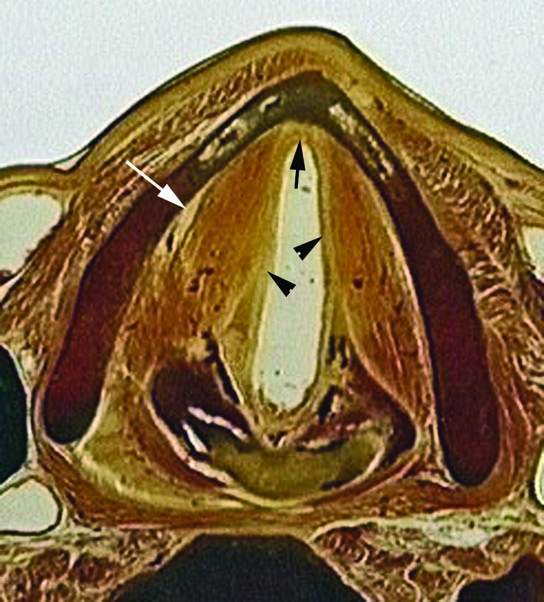
FIGURE 206.9. Whole organ section demonstrating the relationship of the vocal ligaments (arrowheads) to the anterior commissure insertion of the vocal ligaments (arrow). It also demonstrates the fatty paraglottic space (white arrow) between the muscles of the true cord and the thyroid lamina.
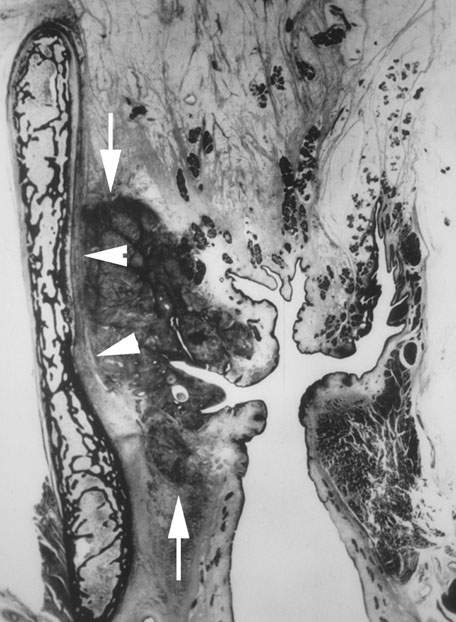
FIGURE 206.10. Coronal whole organ section showing a transglottic cancer spreading from the upper subglottis superiorly to the mid supraglottis (arrows). There is extensive tumor in the paraglottic space, but it does not invade the thyroid lamina (arrowheads).
False Vocal Cord/Ventricle
FVC carcinomas (Figs. 206.6C and 206.7) are usually infiltrative, ulcerative, and endophytic. These cancers invade the paraglottic space early in development and may often spread a considerable distance beneath intact mucosa without producing physical signs (Figs. 6.3, 206.14, and 206.15). This growth pattern leads to understaging at physical examination. These tumors frequently have a broad zone of contact with the perichondrium of the thyroid cartilage early on, but cartilage invasion is a late phenomenon. Spread medially to the lower portion of the infrahyoid epiglottis with invasion of the pre-epiglottic space is uncommon. Paraglottic space submucosal extension to involve the vocal cord can occur, and such vocal cord invasion is often associated with thyroid cartilage invasion. Submucosal extension to the anterior and medial walls of the pyriform sinus occurs early. Subglottic extension occurs only in advanced lesions.
Aryepiglottic Fold/Arytenoid
Early lesions in aryepiglottic fold/arytenoid (Figs. 206.6C and 206.14) may be entirely exophytic. As the lesions enlarge, they invade the cricoarytenoid joint and eventually cause fixation of the TVC. Advanced lesions grow in the paraglottic space to invade the thyroid, epiglottic, and cricoid cartilages (Figs. 206.16 and 206.17). Eventual spread to the base of the tongue and pharyngeal wall may occur via these submucosal pathways. Since the medial wall of the pyriform sinus and aryepiglottic fold are essentially common structures, it may be difficult to determine where the lesion started. Spread to the pyriform sinus apex identifies it as a pyriform sinus cancer. Spread to the remaining supraglottis superiorly and fat in the aryepiglottic fold suggests a marginal supraglottic (aryepiglottic fold) primary.
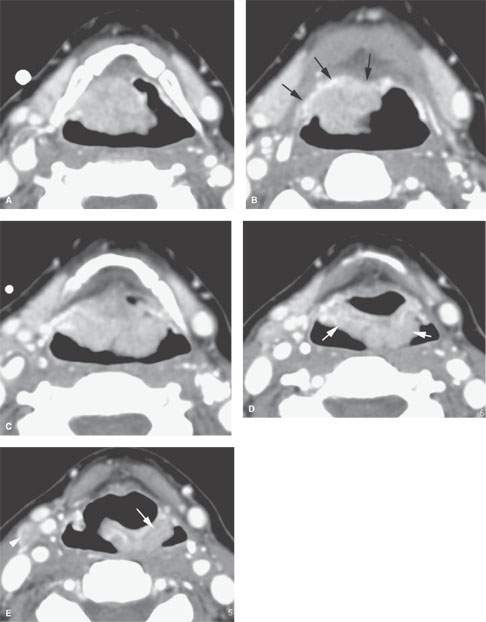
FIGURE 206.11. Contrast-enhanced computed tomography scan of a patient with cancer of the suprahyoid epiglottis. A: This bulky exophytic lesion encompasses the suprahyoid epiglottis. B: The tumor spills superiorly from (A) into vallecula to involve but not invade the tongue base mucosa (arrows). C: Tumor extends to the base of the suprahyoid epiglottis in an exophytic plaquelike manner. D: Tumor continues inferiorly along the pharyngoepiglottic folds and upper aryepiglottic folds and in (E) continues inferiorly along the aryepiglottic fold (arrow).
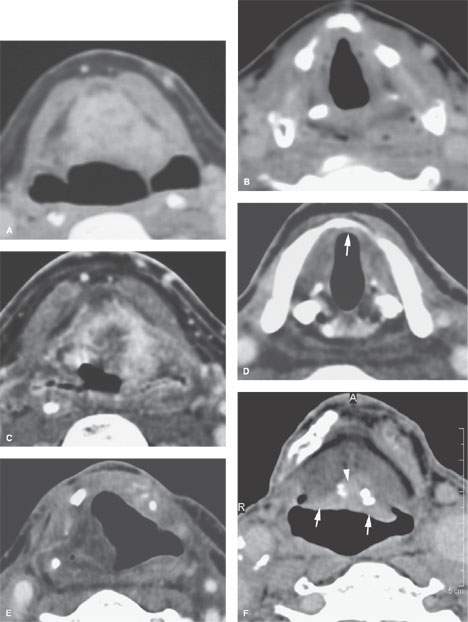
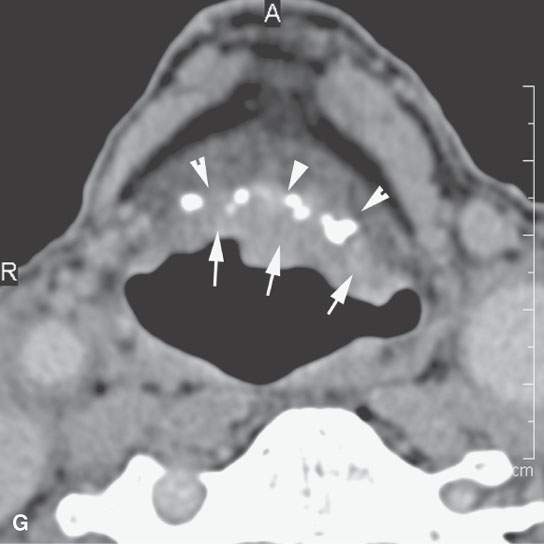
FIGURE 206.12. Two patients with carcinomas of the infrahyoid epiglotis A–E: Contrast-enhanced computed tomography (CECT) study in Patient 1 with deeply infiltrating carcinoma of the infrahyoid epiglottis. In (A), the tumor is endophytic nearly entirely and filling the pre-epiglottic space. In (B), the tumor ended well above the anterior commissure, and the patient was suitable for supraglottic laryngectomy. In (C), the patient elected radiotherapy (RT). A baseline study done about 3 months after the completion of RT showed little response of the tumor (C). In (D), the persistent tumor still ended well above the anterior commissure, so the patient was suitable for supraglottic laryngectomy. In (E), the patient status post supraglottic laryngectomy shows gross total resection. The patient was cured. F, G: CECT study of Patient 2 with an exophytic plaquelike lesion of the infrahyoid epiglottis to contrast with the lesion in (A) through (E). In (F) and (G), at the junction of the infrahyoid and suprahyoid epiglottis, there is plaquelike tumor along the laryngeal surface (arrow). There is minimal invasion of the pre-epiglottic space through the calcified epiglottic cartilage (arrowhead). G: Somewhat more inferiorly the tumor spread along the infrahyoid epiglottis is mainly exophytic. There is no significant penetration of tumor through the calcified epiglottic cartilage into the preepiglottic space (arrowheads). (Note: This second cancer was very suitable for supraglottic laryngectomy or radiation cure. The patient had severe lung disease and was radiated and cured.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree