Fig. 18.1
The electromagnetic spectrum (EMR)
18.3 Spontaneous and Stimulated Emission of Radiation
Electrons surrounding an atom or molecule can exist in more than one energy level but are usually found at the lowest energy level or resting state where they are stable. Energy can be absorbed or lost from a molecule by a photon resulting in a change of the energy level of the electron. An electron in the resting state can absorb a photon of light of the correct wavelength changing it into an excited state. It is unstable in the excited state and will usually drop back down to the resting state, releasing a photon of energy. This process is called spontaneous emission (Fig. 18.2a). If another photon of the appropriate wavelength collides with the electron while it is still excited, the electron will return to its resting state by emitting two photons which are synchronised in time and space and are of the same energy. This is called stimulated emission of radiation (Fig. 18.2b). The emitted photons may stimulate further emissions from excited atoms of the same type. Stimulated emission is rare as normally the majority of electrons are in a resting state. To increase the likelihood of stimulated emission there must be ‘population inversion’. This is a state when photons have a higher probability of encountering excited electrons and stimulating further emissions of photons with the same energy. An external source of energy must be used to increase the proportion of excited atoms in the population to a level where stimulated emission is a frequent occurrence. This process is called ‘pumping’.
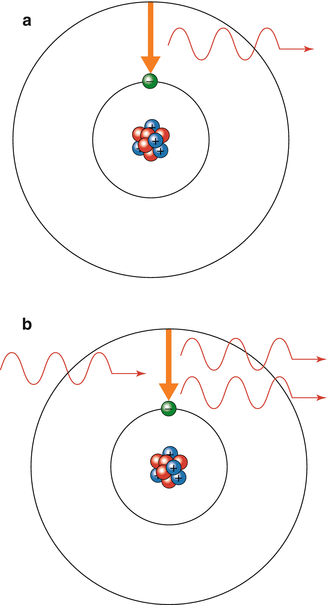

Fig. 18.2
(a) Spontaneous emission. (b) Stimulated emission
Constructing a laser device consists of three basic elements: a pumping system, lasing medium and an optical cavity. The pumping system or the external source of energy creates a population inversion for the excited electrons in the laser chamber. For example, the high-powered flashlamp is used in some dye lasers. The lasing medium or gain is the source of laser radiation and supplies the electrons needed for the stimulated emission of radiation. The wavelength of the emitted light is determined by the distinctive energy transitions of the molecules of the medium. The medium can be gaseous (argon, CO2, copper vapour), liquid (organic dye) or solid (ruby, alexandrite, Er:YAG, Nd:YAG and diode). The optical cavity is a chamber consisting of two parallel mirrors enclosing the laser medium which is excited by the pumping system. Photons of energy in the axis of the chamber are reflected off the mirrors at either end, stimulating further emissions in the same axis. One mirror in the chamber is partially reflective, and this allows some of the stored photons to escape and form a laser beam.
18.4 Properties of Laser Light
Laser light has several unique properties which distinguish it from other types of light such as sunlight. These properties are monochromaticity, coherence and collimation (Stratiagos and Dover 2000).
18.4.1 Monochromaticity
Laser light contains a single wavelength of light or a very narrow band of wavelengths. The wavelength is determined by the lasing medium used in the laser. Other sources of light emit a wide spectrum of wavelengths.
18.4.2 Coherence
Laser radiation waves are in phase with respect to space and time with minimal degree of divergence. This is based on the principle of stimulated emission.
18.4.3 Collimation
Laser waves are highly ordered and parallel which allow the beam to travel long distances without spreading and to be focused on a small spot with very high-power density.
18.5 Radiometry
It is important to understand energy, fluence, power and irradiance. This will help to understand laser physics and laser-tissue interactions. Energy is the capacity to do work and is measured in joules. The energy of laser light refers to the number of photons delivered in a single pulse and is measured in joules and is most suitable to describe the energy of pulsed lasers. Fluence is the energy per area and is also known as energy density which is expressed as joules per cm2. Power is the amount of energy released over time and is measured in watts (J/s). Watts are mainly used to describe continuous wave lasers. Irradiance describes the power density, i.e. the watts per square centimetre. It describes the intensity of a continuous wave laser.
18.6 Laser-Time Dependence
Laser light can be divided into three main types: continuous wave, quasi-continuous wave and pulsed. Continuous wave lasers emit a continuous beam at a constant power (Fig. 18.3a). CO2, argon, krypton and argon dye lasers are examples of continuous wave lasers. Copper vapour is an example of a quasi-continuous wave laser. It delivers a beam with a train of pulses closely spaced together (Fig. 18.3b). Pulsed laser beams are either short pulse with nanosecond (10−9 s) pulse durations or long pulse with pulse durations within milliseconds (10−3 s). Pulse lasers are high-power lasers which emit ultrashort single pulses with extremely high energies (Fig. 18.3c). Quality switching or (Q-switched) lasers contain a photooptical switch or shutter within the laser cavity. This creates a sudden population inversion in the lasing medium and allows the release of extremely short powerful bursts of high energy light within the nanosecond range.
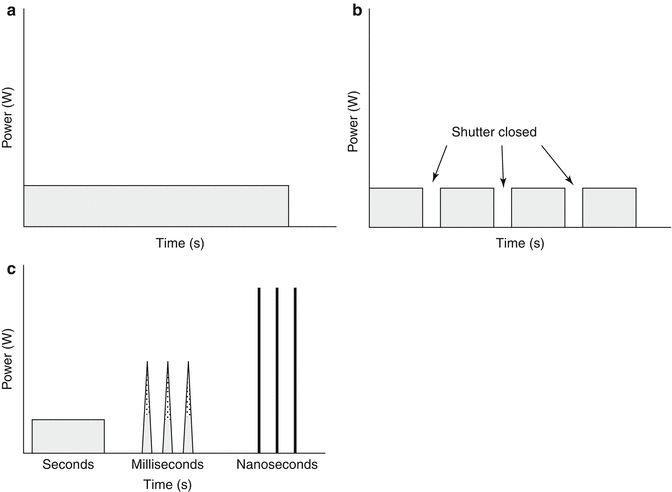

Fig. 18.3
(a) continuous wave lasers. (b) quasi continuous wave lasers. (c) pulsed lasers
18.7 Laser Parameters
18.7.1 Wavelength
The wavelength of the laser light must correspond to the absorption spectrum of the intended target chromophore.
18.7.2 Pulse Duration
The pulse duration is also called the pulse width. This must be equal to or shorter than the thermal relaxation time (TRT) which is directly proportional to the square size of the chromophore.
18.7.3 Energy Density
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree