LATERAL COMPARTMENT: VASCULAR CONDITIONS INCLUDING PENETRATING AND IATROGENIC INJURIES
KEY POINTS
- Magnetic resonance imaging and computed tomography provide the critical and usually definitive data needed in the diagnosis and management of lateral compartment vascular-related diseases.
- Prompt and accurate imaging can help to avoid potentially severe neurologic compromise in carotid disease.
INTRODUCTION
Vascular conditions of the lateral compartment discussed in this chapter include principally acquired conditions of the carotid sheath vessels and vascular malformations. The latter predominantly are malformations of the venolymphatic variety and are discussed more generally in Chapter 9. Some of these conditions overlap with inflammatory disease since a vasculitis or thrombophlebitis may be the cause or effect of an inflammatory lateral compartment condition (Chapter 155).
Clinical Presentation
The clinical presentations generally fall into one of several categories of neck masses, including the following:
- Nonpulsatile neck mass that may be compressible or fluctuates in size
- Pulsatile neck mass, possibly vascular or possibly transmitted pulsation
- Inflammatory (tender) neck mass/process, possibly pulsatile
- Acutely enlarging painful neck mass due to bleeding
- Penetrating trauma
- Surgical complication
APPLIED ANATOMY
The anatomy of the lateral compartment is essentially that of the carotid sheath and its relationship to the anterior and posterior triangles. The relationship to the retropharyngeal space medially and the thoracic inlet and suprahyoid neck space must be understood. Essentially, there is relatively free communication within the lateral compartment between the anterior and posterior triangles and the retropharyngeal space. The relationship of those spaces to the superficial fascia (platysma) and investing and prevertebral layers of the cervical fascia should be reviewed, if necessary, in Chapter 149.
For penetrating trauma, the neck is divided into three anatomic zones. Each zone has vital structures that if injured might alter trauma management. Those zones are as follows:
 Zone 1: Between the clavicle and suprasternal notch and the cricoid cartilage, with structures of interest including the proximal common carotid, vertebral, and subclavian arteries and the trachea, esophagus, thoracic duct, and thymus glands as well as the lower portions of the thyroid and parathyroids
Zone 1: Between the clavicle and suprasternal notch and the cricoid cartilage, with structures of interest including the proximal common carotid, vertebral, and subclavian arteries and the trachea, esophagus, thoracic duct, and thymus glands as well as the lower portions of the thyroid and parathyroids
 Zone 2: Between the cricoid cartilage and the angle of the mandible, with structures of interest including the internal and external carotid arteries, jugular vein, pharynx, larynx, esophagus, recurrent laryngeal nerve, spinal cord, and trachea as well as the upper portions of the thyroid and parathyroids
Zone 2: Between the cricoid cartilage and the angle of the mandible, with structures of interest including the internal and external carotid arteries, jugular vein, pharynx, larynx, esophagus, recurrent laryngeal nerve, spinal cord, and trachea as well as the upper portions of the thyroid and parathyroids
 Zone 3: Between the angle of the mandible and the base of the skull, with structures of interest including the distal extracranial carotid and vertebral arteries and the uppermost segments of the jugular vein
Zone 3: Between the angle of the mandible and the base of the skull, with structures of interest including the distal extracranial carotid and vertebral arteries and the uppermost segments of the jugular vein
Structures of Interest
The analysis of a vascular condition of the lateral compartment depends on a thorough understanding of their relationship to the carotid sheath and the following boundaries:
 Superiorly: Hyoid bone as the arbitrary boundary
Superiorly: Hyoid bone as the arbitrary boundary
 Inferiorly: Thoracic inlet
Inferiorly: Thoracic inlet
 Anteriorly: Anterior triangle
Anteriorly: Anterior triangle
 Posteriorly: Posterior triangle
Posteriorly: Posterior triangle
 Medially: Retropharyngeal space
Medially: Retropharyngeal space
 Laterally: Sternocleidomastoid muscle
Laterally: Sternocleidomastoid muscle
IMAGING APPROACH
Computed Tomography and Magnetic Resonance Imaging
The infrahyoid neck is mainly evaluated with computed tomography (CT) and magnetic resonance imaging (MRI). The specifics and relative value of using these studies in this anatomic region are reviewed in Chapter 149. Problem-driven protocols for CT and MRI are presented in Appendixes A and B. In general, CT with computed tomographic angiography (CTA) is more definitive than MRI for vascular inflammatory conditions of the carotid sheath and is especially useful in the evaluation of penetrating trauma where it is now the principal screening test for Zone 1 and Zone 3 injuries. MRI is preferable when the known or suspected diagnosis is a venolymphatic malformation.
Other
Ultrasound has a potential triage role to play in the evaluation of a pulsatile mass but is most often cost additive and unlikely to contribute to definitive medical decision making. It may actually delay a more timely use of a likely more definitive CT or MRI study; such delay can be costly in the case of acute penetrating injury. It may be used to screen in Zone 2 penetrating injuries.
Catheter angiography is use selectively and most often as a prelude to endovascular intervention once the diagnosis has been established by imaging and related CTA or magnetic resonance (MR) angiography.
PATHOPHYSIOLOGY AND PATTERNS OF DISEASE AND DIFFERENTIAL DIAGNOSIS AS SEEN ON MAGNETIC RESONANCE IMAGING AND COMPUTED TOMOGRAPHY
The initial evaluation of a potential vascular process in the lateral compartment is highly driven by the patient age, specific clinical situation, and related likely disease category; therefore, this will be used as a framework for this discussion.
Developmental Vascular Malformations
Venolymphatic Malformations and Others
The embryology of the lymphatic system explains the pathogenesis and imaging appearance of cervicothoracic (and other) venolymphatic malformations of the region. This embryology and the resulting gross morphologic and related imaging variations are summarized in Chapter 9. The modes of clinical presentation and general patient demographics are also presented in Chapter 9. In this chapter, the following background information related to their presentations in the compartments of the neck and at the thoracic inlet is summarized.
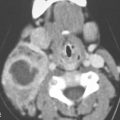
Cervical–thoracic lymphatic development begins a short time after arterial development, when the venous lymphatic primordia endothelial buds sprout from the veins and coalesce to form plexuses. This explains why blood vessel elements are frequently mixed with abnormal lymphatic spaces1 (Fig. 154.1).
The plexuses then form paired jugular sacs, while the paired axillary lymph sacs emerge independently. The axillary and jugular sacs enlarge rapidly, each having a single communication with each other independently as well as with their respective internal jugular vein. These connections widen and become impossible to distinguish from the original two primordia. Growth of the juguloaxillary sac continues cranially and posterolaterally. The continued growth is mainly between the trapezius and sternocleidomastoid muscles, where it eventually reaches the subcutaneous area of the posterior triangle of the neck. This later developmental phase extension may explain a tendency for a venolymphatic malformation to more commonly present posterior to the carotid sheath (Fig. 154.2). Meanwhile, the thoracic duct primordia also mature.
The tendency for all growth of these venolymphatic malformations is to involve the spaces predominantly deep to the superficial fascia and insinuate along the vessels from which they arise. In this way, the malformations also follow the cervical nerves supplied by the malformation finding risk(s) along their normal path. This includes the possibility of following those neurovascular structures across fascial boundaries as well as around and into the various viscera of the neck that those neurovascular bundles would supply (Fig. 154.3).
By such developmental tendencies, isolated relatively simple malformations as well as highly complex transcompartmental malformations become possible and their growth patterns relatively easy to understand (Figs. 154.1–154.3). For instance, an isolated cystic hygroma may form from lost connections between the primordial sac and its bud, leading to development of an isolated hygroma in the posterior triangle region in a patient with an otherwise normal lymphatic system (Fig. 154.2). Vascular lymphatic malformations, composed of both lymphatic and vascular elements, can also be understood mechanistically. A venolymphatic malformation such as that seen in Figure 154.1 may be explained by a failure of the right paratracheal and internal thoracic lymph plexuses to join with the juguloaxillary sac. The mixed vascularity is likely due to an abnormal bud retaining its original venous communication, not allowing the related mesenchyme of the venous bud to fully differentiate into a purely lymphatic structure. The malformation in that way grows while retaining some blood vessel vascular characteristics.



FIGURE 154.1. Six patients with venolymphatic malformations with mixed cystic and vascular features other than cystic components such as fluid levels and vascular components that help to identify the etiology of these lesions as indicated by arrows. Contrast-enhanced computed tomography studies were done in the first four patients. (These VLMs tend to grow transcompartmental.) A–D: Patient 1 with a relatively superficial bilateral venolymphatic malformation extending from the upper neck to the supraclavicular fossa. E: Patient 2. A transcompartmental venolymphatic malformation involving the floor of the mouth and lateral neck. F: Patient 3 showing multifocal vascular malformations, some with dominant blood vessel components. G, H: Patient 4 showing multifocal mixed venolymphatic malformations and one particularly unusual malformation in the retropharyngeal space in addition to the lateral compartments. Also note the vascular malformations with blood vessel components in both parotid glands in (H). I, J: Patients 5 and 6 with ultrasound done for the initial evaluation. While the ultrasound shows cystic and vascular elements to confirm the clinical impression of the venolymphatic malformation, it did not show the full extent of the malformations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree