(1)
Department of Radiology Chair, Central State Medical Academy Department of Radiology, Moscow, Russia
ABVS technology has some limitations. Automated breast ultrasound is limited in women with macromastia and pronounced ptosis [1, 2]. Isobe et al. [3] pointed out some difficulties in the scanning of large breasts and the retroareolar area despite the large scanning surface. Furthermore, they presume that even with optimal scanning technique, the peripheral areas of the breast parenchyma are not fully covered by ABVS [3]. Therefore, some areas of the breast, such as deep lateral areas, do not have proper visualization and complete coverage using ABVS. The scanning field of 17 cm does not allow the inclusion of the entire volume of the breast in a single scan for patients with a bra cup size (F) or more (Fig. 5.1). This reduces the diagnostic value of ABVS when compared with conventional two-dimensional ultrasound [3]. In our view this arises only with coronal scans, in which the outer portions of the gland are not so compressed and could not be evaluated so thoroughly, but we used some special projections in which a patient lies on her side for scanning the lateral portion of the gland or shifting the breast laterally for scanning medial portions of the gland. Similarly we use the technique of shifting the breast down for scanning the upper part and upward for scanning the inferior part. If a mass is detected in the lower or inner quadrants on the mammogram, it is possible to supplement the study by scanning from the mediolateral view or separately capture the lower quadrants. These scanning principles will result in better visualization of the tissues of the subsequent zones [4]. Therefore, we recommend in such cases to follow the principle of sequential study of all zones of interest with maximum capture of all areas and obtaining additional views of quadrants not included in the initial view.


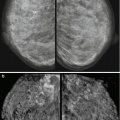
Fig. 5.1
Side-by-side comparison of the X-ray mammography craniocaudal direct view and R SUP (superior-to-inferior view) ABVS images of the right breast in a patient with macromastia. The ABVS transducer covers only half of the field covered by MMG
There is no experience in examining the axillary region with ABVS, although it is of special importance in breast cancer diagnosis. Today, sentinel node biopsy is the standard therapy for women at the preoperative stage with a negative nodal status, which requires ultrasound of the axilla [5]. Furthermore, lymph node alterations may be the first sign in mammographically and/or sonographically occult breast cancer or other malignant diseases. Therefore, additional conventional ultrasound of the axilla would be necessary after a suspicious ABVS scan. This drawback of ABVS is noted by all researchers, and this limits the possibility of using this method for screening [6–14].
In our clinical experience, we faced a case of false-negative diagnosis of multifocal breast cancer localized in the axillary process. Retrospective analysis of the whole array of stored ultrasound data showed that the tumor was located outside the scanning field. It was impossible to cover the axillary process completely at a volume scan, and therefore we could not view the tumor by processing the entire array of 3D data (Fig. 5.2). To avoid such false-negative cases, the patient should initially be examined with conventional 2D ultrasound followed by an ABVS study with additional scanning of the breast’s axillary process, which may reveal multifocal tumor growth in the patient.




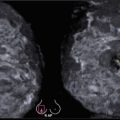
Fig. 5.2
Multifocal IDC (3T1N0M0, Luminal B, G2) with localization in the axillaris process that was missed on ABVS due to the peripheral location. Comparison of HHUS, ABVS, and MMG. (a) Multiple hypoechoic tumors with indistinct margins with a hyperechoic halo sign and increased anteroposterior diameter. (b) Sonoelastography of the lesions. Increased stiffness of both tumors, colored deep blue. (c) Bilateral ABVS in coronal anteroposterior projection. A symmetrical distribution of the breast parenchyma, absence of additional lesions, and retraction phenomenon. (d, e) Side-by-side comparison of mammographic and ABVS images in the corresponding projections. (d) Direct CC MMG (lower part) and R SUP ABVS (upper part). (e) MLo MMG (right part) and LMO ABVS (left part)
Additionally, shadowing artifacts occur in the retroareolar region despite the special algorithm (adaptive nipple shadow reduction tool) used for reduction of nipple shadowing and to a certain extent in the remaining breast volume. Therefore, a certain proportion of breast parenchyma may be lost in the volume data. This may reduce the diagnostic potential in comparison to handheld ultrasound. As previously mentioned, a special technique for acquiring volumetric data was suggested. Maximum lateralization of the nipple is used to avoid these artifacts and reduces no-show zones (Fig. 5.3).


Fig. 5.3
An example of a nipple shadow artifact. A large anechoic zone is seen around the nipple in the L Lat scan (left latero-medial oblique) scan. Note the nipple is not properly lateralized; this results in the large “no-show” zone
During an automatic scan, movement or conversation artifacts arise in some cases, which adversely affect the perception of 3D data (Figs. 5.4 and 5.5). The lack of contact of the scanning membrane with the skin of the gland can cause some artifacts in the so-called “dumb” zone, such as in scar deformity of the breast after lumpectomy or sectoral resection, in severe retraction of the nipple and breast deformation in infiltrating breast cancer, in breast implants, in expanders, and sometimes in cases of severe pain in mastitis (Fig. 5.6). In these cases, you should conduct an examination using the standard 2D technique.


Fig. 5.4




An example of an artifact from a movement during acquisition of the 3D data. A large strip line is seen in the upper portion of the left breast obtained in the latero-medial position (white arrow). Small waves are also seen above that line; these also result from speaking during the acquisition (arrowheads)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree